261394
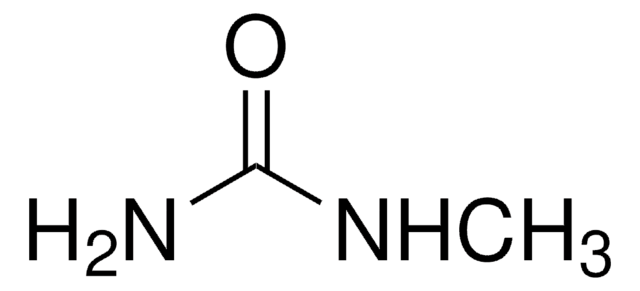
1,1-Dimethylurea
99%
Synonyme(s) :
N,N-Dimethylurea
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
(CH3)2NCONH2
Numéro CAS:
Poids moléculaire :
88.11
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
99%
Forme
solid
Pf
178-183 °C (lit.)
Solubilité
water: soluble 5%, clear, colorless
Groupe fonctionnel
amine
Chaîne SMILES
CN(C)C(N)=O
InChI
1S/C3H8N2O/c1-5(2)3(4)6/h1-2H3,(H2,4,6)
Clé InChI
YBBLOADPFWKNGS-UHFFFAOYSA-N
Informations sur le gène
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Description générale
Nonlinear optical properties of 1,1-dimethylurea (N,N′ dimethylurea), have been evaluated through second-harmonic generation.
Application
1,1-Dimethylurea (N,N-dimethylurea) has been used in the Dowex-50W ion exchange resin-promoted synthesis of N,N′-disubstituted-4-aryl-3,4-dihydropyrimidinones.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
S Sandler et al.
Diabetologia, 23(4), 374-378 (1982-10-01)
The protective effect on streptozotocin-induced diabetes of dimethyl urea, a hydroxyl radical scavenger, has been evaluated in vivo and in vitro. Pretreatment with dimethyl urea before a single diabetogenic dose of streptozotocin partially protected NMRI mice from hyperglycaemia, whereas the
Nonlinear optical properties of N, N' dimethylurea.
Halbout JM, et al.
Applied Physics Letters, 37(10), 864-866 (2008)
G L Wilson et al.
Diabetologia, 27(6), 587-591 (1984-12-01)
In studies to evaluate possible inhibitors of the B-cell toxin, streptozotocin, the superoxide scavenger, superoxide dismutase, did not prevent or reduce the toxic effects of streptozotocin as determined by loss of insulin secretion from rat pancreatic B cells in monolayer
G P Meshram et al.
Mutation research, 279(4), 275-280 (1992-06-16)
Methyl isocyanate (MIC) in aqueous solution forms methylamine (MA) and N,N'-dimethylurea (DMU). MA in buffered system further converts into its salt form, methylamine hydrochloride (MAH). Therefore, MAH and DMU were evaluated for their mutagenic activity in the in vitro Ames
W J Caspary et al.
Mutation research, 174(4), 285-293 (1986-08-01)
Methylisocyanate (MIC) induced mutagenic responses in the absence of exogenous activation in the mouse lymphoma cell forward mutation assay at concentrations as low as 8-24 microM. MIC produced predominantly small mutant colonies, suggesting the possibility of clastogenic activity. The intermediate
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique