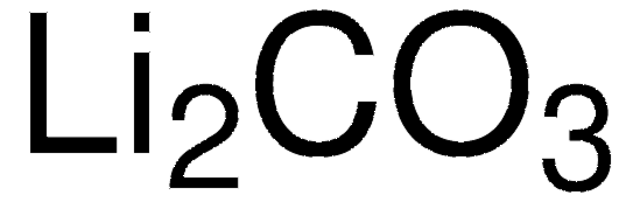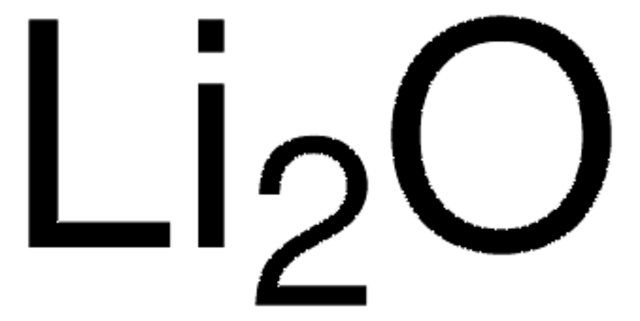229733
Bromure de lithium
powder and chunks, ≥99.995% trace metals basis
Synonyme(s) :
Lithium monobromide
About This Item
Produits recommandés
Qualité
for analytical purposes
Essai
≥99.995% trace metals basis
Forme
powder and chunks
Impuretés
≤50.0 ppm Trace Metal Analysis
Pf
550 °C (lit.)
Application(s)
battery manufacturing
Chaîne SMILES
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
Clé InChI
AMXOYNBUYSYVKV-UHFFFAOYSA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Space cooling using geothermal single‐effect water/lithium bromide absorption chiller: This research explores the use of lithium bromide in geothermal absorption chillers for space cooling applications (M El Haj Assad, M Sadeghzadeh, 2021).
- A facile and fast method for quantitating lignin in lignocellulosic biomass using acidic lithium bromide trihydrate (ALBTH): The paper introduces a novel method for lignin quantification using lithium bromide trihydrate (N Li, X Pan, J Alexander, 2016).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique