151874
Sulfoxyde de diméthyle-d6, Diméthylsulfoxyde-d6
99.9 atom % D
Synonyme(s) :
(Sulfoxyde de méthyle)-d6, (Méthylsulfoxyde)-d6, DMSO-d6, Hexadeutérodiméthyl sulfoxyde, Hexadeutérodiméthylsulfoxyde
About This Item
Produits recommandés
Pression de vapeur
0.42 mmHg ( 20 °C)
Niveau de qualité
Pureté isotopique
99.9 atom % D
Essai
99% (CP)
Forme
liquid
Température d'inflammation spontanée
573 °F
Limite d'explosivité
42 %
Technique(s)
NMR: suitable
Impuretés
≤0.0250% water
water
Indice de réfraction
n20/D 1.476 (lit.)
pb
189 °C (lit.)
Pf
20.2 °C (lit.)
Densité
1.190 g/mL at 25 °C (lit.)
Changement de masse
M+6
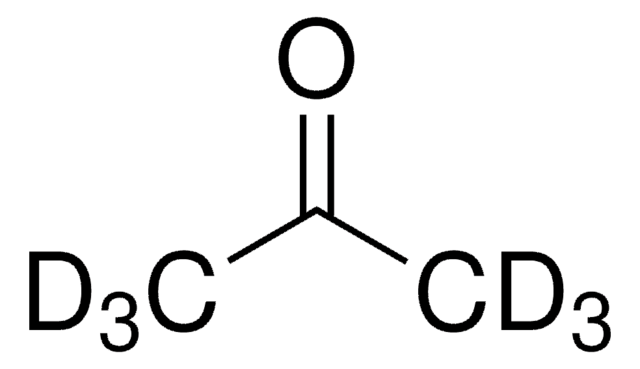
Chaîne SMILES
[2H]C([2H])([2H])S(=O)C([2H])([2H])[2H]
InChI
1S/C2H6OS/c1-4(2)3/h1-2H3/i1D3,2D3
Clé InChI
IAZDPXIOMUYVGZ-WFGJKAKNSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Investigation of the Spatial Structure of Flufenamic Acid in Supercritical Carbon Dioxide Media via 2D NOESY : cette recherche explore les interactions moléculaires de l'acide flufénamique dans du CO₂ supercritique, en mettant en avant des techniques analytiques basées sur l'utilisation du diméthylsulfoxyde-d₆ comme solvant pour améliorer les lectures spectrales (Khodov et al., 2023).
- Taguchi Approach for Optimization of a Green Quantitative 1H-NMR Practice for Characterization of Levetiracetam and Brivaracetam in Pharmaceuticals : emploie le diméthylsulfoxyde-d₆ en spectroscopie RMN pour optimiser les méthodes analytiques de caractérisation des produits pharmaceutiques, en privilégiant l'écoresponsabilité (Mansour et al., 2022).
- Counterintuitive torsional barriers controlled by hydrogen bonding : analyse la torsion moléculaire modulée par les liaisons hydrogène, en exploitant le diméthylsulfoxyde-d₆ pour explorer les effets du solvant, contribuant à une meilleure compréhension de la dynamique moléculaire en chimie analytique (Barbero et al., 2020).
- Elucidating Interactions between DMSO and Chelate-Based Ionic Liquids : examine la dynamique des interactions entre le diméthylsulfoxyde-d₆ et des liquides ioniques, offrant un aperçu des interactions solvant-soluté, fondamentales dans les méthodologies analytiques (Chen et al., 2015).
- Conformation of an octapeptide fragment (2-9) of kaliocin-1 in DMSO-d6 by 1H NMR and restrained molecular dynamics : cette étude emploie le diméthylsulfoxyde-d₆ en RMN pour découvrir les propriétés conformationnelles d'un peptide, ce qui contribue à mieux comprendre la structure dudit peptide dans des conditions analytiques (Sunilkumar et al., 2007).
Produits recommandés
À utiliser avec
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
190.4 °F
Point d'éclair (°C)
88 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Use this reference table to find the coupling values and chemical shifts of our NMR (deuterated) solvents. Melting and boiling points, molecular weight, density, and CAS number are also listed.
Contenu apparenté
NMR spectroscopy elucidates molecular structure and purity via nuclear spin states in a strong magnetic field.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique