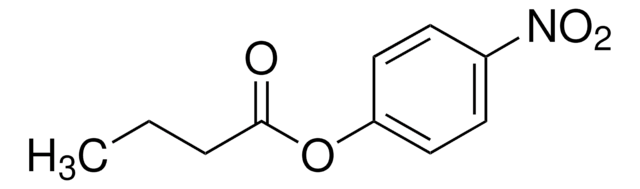
L0382
Lipase from porcine pancreas
Type VI-S, ≥20,000 units/mg protein, lyophilized powder
Synonym(s):
PPL, Triacylglycerol acylhydrolase, Triacylglycerol lipase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
type
Type VI-S
Quality Level
form
lyophilized powder
specific activity
≥20,000 units/mg protein
composition
Protein, 40-70%
shipped in
wet ice
storage temp.
−20°C
InChI
1S/C11H9N3O2.Na/c15-8-4-5-9(10(16)7-8)13-14-11-3-1-2-6-12-11;/h1-7,16H,(H,12,14);/q;+1/b13-9-;
InChI key
QWZUIMCIEOCSJF-CHHCPSLASA-N
Looking for similar products? Visit Product Comparison Guide
General description
Lipase from porcine pancreas (PPL) or porcine pancreatic lipase is a small globular protein with N-terminal α/β type fold. It comprises catalytic triad Ser, Asp, and His residues and a C-terminal β-sandwich domain. PPL belongs to a subclass of the carboxylesterases family.
Application
Lipase from porcine pancreas has been used:
- in lipase inhibition assay with plant extracts
- in in vitro digestion gelatinized starch/fiber mixtures
- with simulated intestinal fluid to mimic the digestive tract condition of by L. fermentum PC1 in fermented oats
Lipases are used industrially for the resolution of chiral compounds and the transesterification production of biodiesel.
Biochem/physiol Actions
Lipase from porcine pancreas (PPL) catalyzes the hydrolysis of fats (lipids). It plays an essential role in the digestion, transport, and processing of dietary lipids (e.g. triglycerides, fats, oils) in most living organisms. PPL is cost-effective and is useful in biotransformation reactions. It displays high selectivity, and catalytic activity. PPL is also used in the detergent formulation for removing oil stains from clothes.
Lipases catalyze the hydrolysis of esters in aqueous solutions and the synthesis of esters in non-aqueous solutions. They also produce hydroxyl and carboxylic groups through the hydrolysis of ester linkages in poly(ethylene terephthalate).
Lipases catalyze the hydrolysis of triacylglycerols into glycerol and free fatty acids.
Lipases catalyze the hydrolysis of triacylglycerols into glycerol and free fatty acids.
Unit Definition
One unit will hydrolyze 1.0 microequivalent of fatty acid from a triglyceride in 1 hr at pH 7.7 at 37 °C using olive oil.
Analysis Note
Protein determined by biuret.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hua Zhao et al.
PloS one, 9(12), e114385-e114385 (2014-12-30)
Pancreatic lipase plays a key role in intestinal digestion of feed fat, and is often deficient in young animals such as weaning piglets. The objective of this study was to express and characterize a partial codon optimized porcine pancreatic lipase
Properties and biotechnological applications of porcine pancreatic lipase
Mendes AA, et al.
Journal of Molecular Catalysis. B, Enzymatic, 78 (2012)
Marcella Denaro et al.
Antioxidants (Basel, Switzerland), 10(2) (2021-01-28)
Recently, several studies have highlighted the role of Citrus flavanones in counteracting oxidative stress and inflammatory response in bowel diseases. The aim of study was to identify the most promising Citrus flavanones by a preliminary antioxidant and anti-inflammatory screening by
Liwei Chen et al.
Frontiers in microbiology, 11, 609734-609734 (2020-12-22)
Lactobacillus fermentum PC1 with proven probiotic properties was used to ferment oats with added honey to develop a probiotic beverage with enhanced bioactive ingredients. The viable Lactobacilli were enumerated during the fermentation and storage at 4°C, as well as after
Yu-Cheng Lin et al.
The American journal of clinical nutrition, 99(4), 869-874 (2014-01-31)
A genome-wide association study identified variants in or near patatin-like phospholipase domain-containing-3 (PNPLA3), neurocan (NCAN), lysophospholipase-like 1 (LYPLAL1), glucokinase regulatory protein (GCKR), and protein phosphatase 1 regulatory subunit 3b (PPP1R3B) that were strongly associated with nonalcoholic fatty liver disease (NAFLD)
Articles
Lipid Induced Insulin Resistance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service