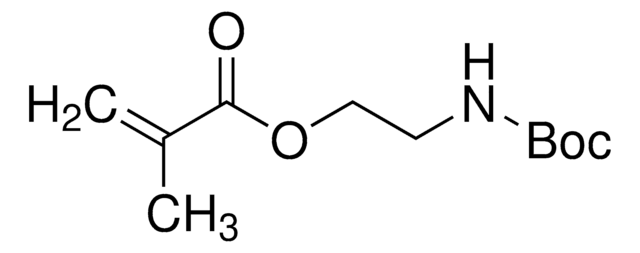
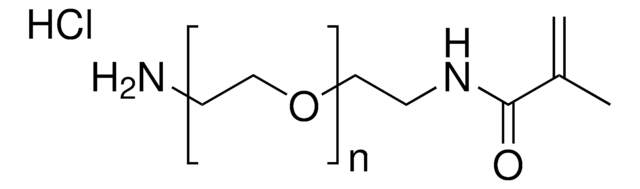
900652
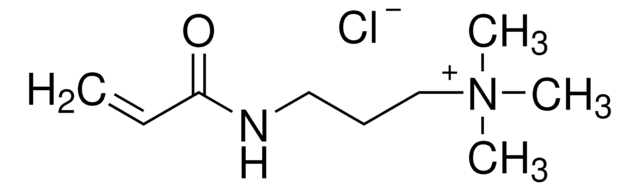
2-Aminoethylmethacrylamide hydrochloride
≥98%
Synonym(s):
N-(2-Aminoethyl) methacrylamide hydrochloride, AEMA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13ClN2O
CAS Number:
Molecular Weight:
164.63
MDL number:
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥98%
form
powder or chunks
mp
120-125 °C
storage temp.
−20°C
SMILES string
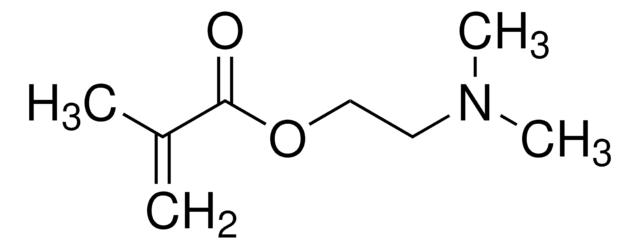
Cl.N(CCN)C(=O)C(=C)C
InChI key
RUSMHXACRXXLKQ-UHFFFAOYSA-N
Related Categories
Application
Monomer for polymerization reactions, may be used to synthesize polymers for nucleic acid complexation and polyplex formation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marya Ahmed et al.
Bioconjugate chemistry, 22(6), 1228-1238 (2011-05-05)
The facile synthesis of biocompatible and nontoxic gene delivery vectors has been the focus of research in recent years due to the high potential in treating genetic diseases. 2-Methacryloxyethyl phosphorylcholine (MPC) copolymers were recently studied for their ability to produce
Wenchen Li et al.
Langmuir : the ACS journal of surfaces and colloids, 30(42), 12619-12626 (2014-09-30)
We report two new amino acid based antifouling zwitterionic polymers, poly(N(4)-(2-methacrylamidoethyl)asparagine) (pAspAA) and poly(N(5)-(2-methacrylamidoethyl)glutamine) (pGluAA). The vinyl monomers were developed from aspartic acid and glutamic acid. Surface-initiated photoiniferter-mediated polymerization was employed to graft polymer brushes from gold surfaces. Different thickness
Diblock glycopolymers promote colloidal stability of polyplexes and effective pDNA and siRNA delivery under physicological salt and serum conditions.
Smith AE, et al.
Biomacromolecules, 12, 3015-3022 (2011)
Wei Sun et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(27), 7701-7707 (2007-06-30)
Linear copolymers have been developed which carry binding sites tailored for sulfated sugars. All binding monomers are based on the methacrylamide skeleton and ensure statistical radical copolymerization. They are decorated with o-aminomethylphenylboronates for covalent ester formation and/or alkylammonium ions for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service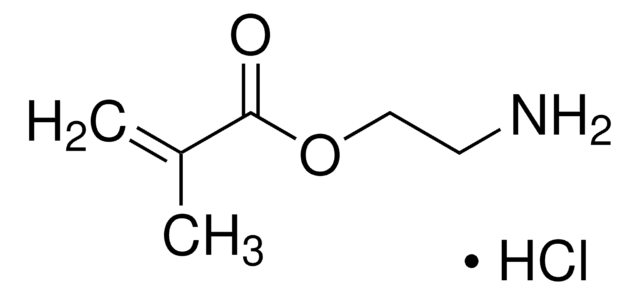
![N-[3-(Dimethylamino)propyl]methacrylamide 99%, contains MEHQ as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/295/145/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951/640/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951.png)