498521
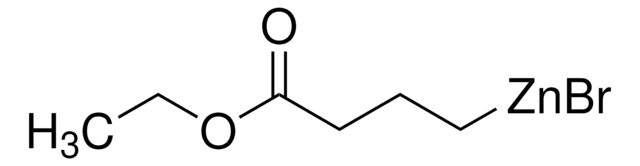
3-Ethoxy-3-oxopropylzinc bromide solution
0.5 M in THF
Synonym(s):
(2-Ethoxycarbonylethyl)zinc bromide, (3-Ethoxy-3-oxopropyl)zinc(II)bromide, 3-Ethoxy-3-oxopropylzinc bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5O2CCH2CH2ZnBr
CAS Number:
Molecular Weight:
246.42
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
0.5 M in THF
density
0.972 g/mL at 25 °C
functional group
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)CC[Zn]Br
InChI
1S/C5H9O2.BrH.Zn/c1-3-5(6)7-4-2;;/h1,3-4H2,2H3;1H;/q;;+1/p-1
InChI key
XLKAROLEWCMBDC-UHFFFAOYSA-M
Application
3-Ethoxy-3-oxopropylzinc bromide is a reagent used to insert ethyl propanoate group to the substrate. It can be used in:
- Synthesis of γ-keto esters from aryl chlorides.
- Synthesis of sulfones from 1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide) (DABSO) and alkyl halides.
- Negishi cross-couplings with aryl vinyl phosphates to synthesize 1,1-disubstituted alkenes.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17.0 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Investigations on the Suzuki? Miyaura and Negishi Couplings with Alkenyl Phosphates: Application to the Synthesis of 1, 1-Disubstituted Alkenes.
Hansen A L, et al.
The Journal of Organic Chemistry, 72(17), 6464-6472 (2007)
Synthesis of the Novel Tetrahydropyrazolo [3, 4-c] pyridin-5-one Scaffold.
Howe N J, et al.
Synlett, 26(02), 228-232 (2015)
Synthesis of sulfones from organozinc reagents, DABSO, and alkyl halides.
Rocke B N, et al.
Organic Letters, 16(1), 154-157 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-(1,3-Dioxolan-2-yl)]ethyl]zinc bromide solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/208/700/ec5d0440-e703-4c3f-80f9-95d43bbef39b/640/ec5d0440-e703-4c3f-80f9-95d43bbef39b.png)