106003
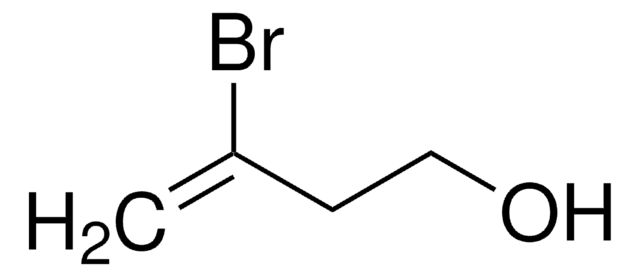
2,3-Dibromopropene
80%, technical grade
Synonym(s):
(2-Bromo-2-propenyl) bromide, 2,3-Dibromopropylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH2BrCBr=CH2
CAS Number:
Molecular Weight:
199.87
Beilstein:
878169
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay:
80%
Recommended Products
grade
technical grade
Quality Level
Assay
80%
refractive index
n20/D 1.544 (lit.)
bp
42-44 °C/17 mmHg (lit.)
density
2.045 g/mL at 25 °C
functional group
bromo
SMILES string
BrCC(Br)=C
InChI
1S/C3H4Br2/c1-3(5)2-4/h1-2H2
InChI key
YMFWYDYJHRGGPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dibromopropene is an intermediate formed during the hydrolysis of nematocide:1,2-dibromo-3-chloropropane.
Application
2,3-Dibromopropene was used to test 2- and 3-carbon halogenated hydrocarbons for mutagenicity for Salmonella typhimurium strain TA 100 . It was used in the synthesis of N(Boc)-L-(2-Bromoallyl)-glycine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S J Stolzenberg et al.
Environmental mutagenesis, 2(1), 59-66 (1980-01-01)
Short-chain, 2- and 3- carbon halogenated hydrocarbons were tested for mutagenicity for Salmonella typhimurium strain TA 100 both with and without the presence of S-9. Without exception, all brominated derivatives were more mutagenic than the chlorinated derivatives, usually by a
N-(Boc)-L-(2-Bromoallyl)-glycine: a versatile intermediate for the synthesis of optically active unnatural amino acids.
Leanna MR and Morton HE.
Tetrahedron Letters, 34(28), 4485-4488 (1993)
Gerald Pratsch et al.
The Journal of organic chemistry, 80(22), 11388-11397 (2015-10-31)
Visible-light photoreductive coupling of 2-arylallyl bromides in the presence of the photocatalyst Ru(bpy)3(PF6)2, a Hantzsch ester, and i-Pr2NEt gives 2,5-diaryl-1,5-dienes in high yield. This method avoids the use of stoichiometric metal reductants and is compatible with the presence of halogen
Kinetics and products of hydrolysis of 1,2-dibromo-3-chloropropane.
N E Burlinson et al.
Environmental science & technology, 16(9), 627-632 (1982-09-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service