679437
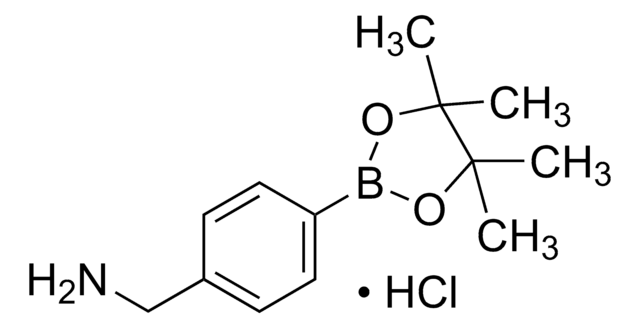
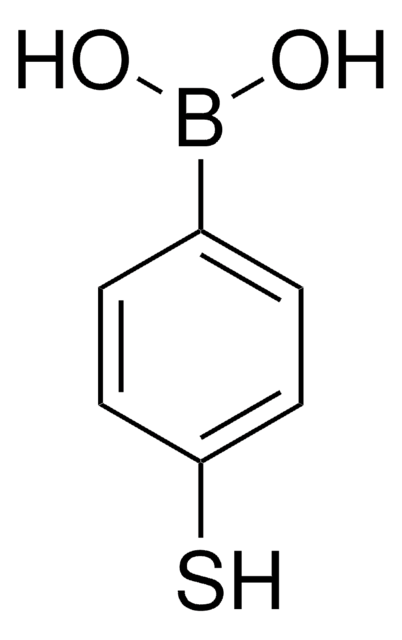
4-(Bromomethyl)phenylboronic acid
Synonym(s):
α-Bromo-p-tolueneboronic acid, p-Boronobenzyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
BrCH2(C6H4)B(OH)2
CAS Number:
Molecular Weight:
214.85
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
mp
173-177 °C
functional group
bromo
storage temp.
2-8°C
SMILES string
OB(O)c1ccc(CBr)cc1
InChI
1S/C7H8BBrO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,10-11H,5H2
InChI key
PDNOURKEZJZJNZ-UHFFFAOYSA-N
General description
May contain varying amounts of anhydride
Application
Reactant involved in:
- Design of boronic acid-based autotaxin inhibitors
- Synthesis of boronated phosphonium salts
- Studies of incorporation of boronic acid groups to enhance gene transfection capability
- Investigations of the effect of boronic acid-positioning in optical glucose-sensing ensemble
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chongyi Liu et al.
Advanced healthcare materials, 5(5), 584-592 (2016-01-21)
Cationic dendrimers are widely used as nonviral gene vectors, however, current gene materials based on dendrimers are either little effective or too toxic on the transfected cells. Here, a facile strategy is presented to prepare high efficient dendrimers with low
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service