U104
UK 14,304
Synonym(s):
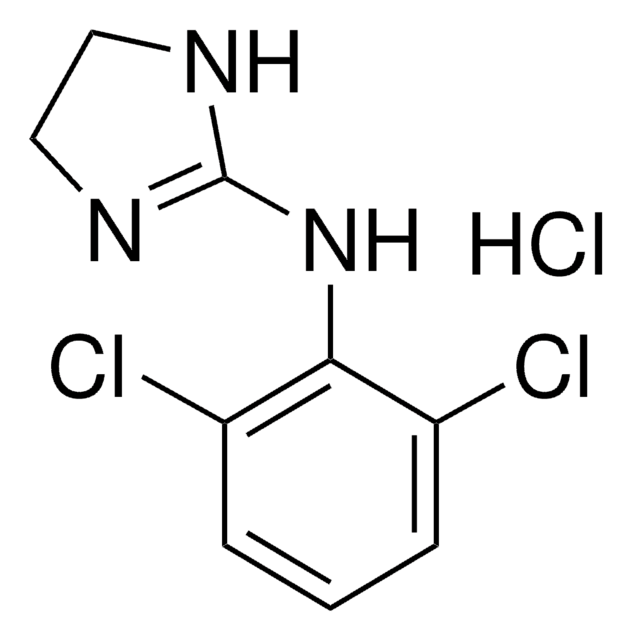
5-Bromo-N-(2-imidazolin-2-yl)-6-quinoxalinamine, 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine, Brimonidine
About This Item
Recommended Products
Quality Level
solubility
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: <0.8 mg/mL
DMSO: >6.5 mg/mL
ethanol: <8 mg/mL (warm)
H2O: insoluble
SMILES string
Brc1c(NC2=NCCN2)ccc3nccnc13
InChI
1S/C11H10BrN5/c12-9-7(17-11-15-5-6-16-11)1-2-8-10(9)14-4-3-13-8/h1-4H,5-6H2,(H2,15,16,17)
InChI key
XYLJNLCSTIOKRM-UHFFFAOYSA-N
Gene Information
human ... ADRA1A(148) , ADRA2A(150) , ADRA2B(151) , ADRA2C(152)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Features and Benefits
Preparation Note
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about alpha-2 adrenoceptor and its subtypes, mediated responses, and applications of agonists. Included is a list of available products and a comparison table.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service