U101
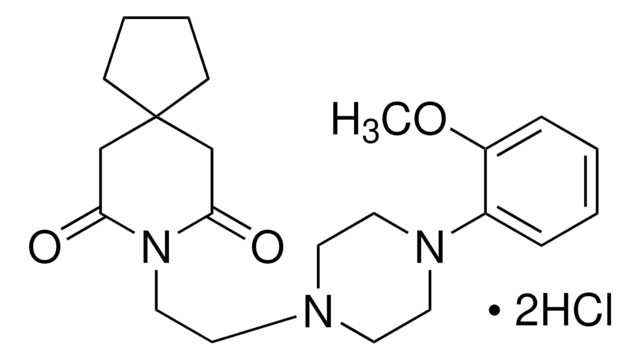
5-Methylurapidil
solid
Synonym(s):
5-Methyl-6[[3-[4-(2-methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil
About This Item
Recommended Products
form
solid
Quality Level
color
white
solubility
H2O: 0.4 mg/mL
0.1 M HCl: 3.8 mg/mL
SMILES string
Cl[H].COc1ccccc1N2CCN(CCCNC3=C(C)C(=O)N(C)C(=O)N3C)CC2
InChI
1S/C21H31N5O3.ClH/c1-16-19(23(2)21(28)24(3)20(16)27)22-10-7-11-25-12-14-26(15-13-25)17-8-5-6-9-18(17)29-4;/h5-6,8-9,22H,7,10-15H2,1-4H3;1H
InChI key
WAZDYFHTLYHMKO-UHFFFAOYSA-N
Gene Information
human ... ADRA1A(148) , ADRA1B(147) , ADRA1D(146)
Application
Biochem/physiol Actions
Features and Benefits
Preparation Note
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Review alpha 1 adrenoceptors as well as their agonists, antagonists, and tissue expression patterns. We suggest several modulators and alternatives for working with a-1 adrenoreceptors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service