I2753
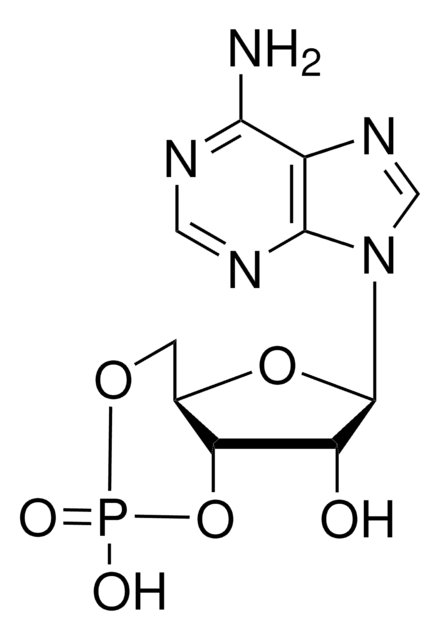
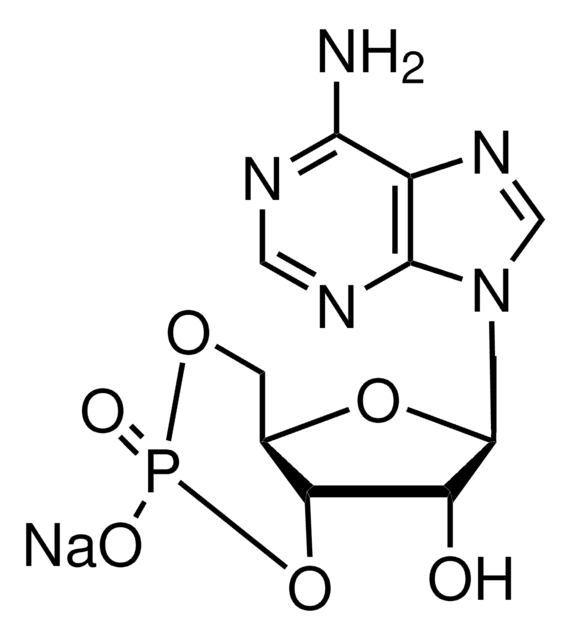
Inosine 3′:5′-cyclic monophosphate sodium salt
≥98%
Synonym(s):
cIMP
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H10N4O7PNa
CAS Number:
Molecular Weight:
352.17
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥98%
form
powder
storage temp.
−20°C
SMILES string
[Na].OC1C2OP(O)(=O)OCC2OC1n3cnc4C(=O)N=CNc34
InChI
1S/C10H11N4O7P.Na.H/c15-6-7-4(1-19-22(17,18)21-7)20-10(6)14-3-13-5-8(14)11-2-12-9(5)16;;/h2-4,6-7,10,15H,1H2,(H,17,18)(H,11,12,16);;
InChI key
XIUBZBACWHALEW-UHFFFAOYSA-N
Application
Inosine 3′:5′-cyclic monophosphate (cIMP) is used in studies that compare the substrate specificity and activity of cyclic purines, including the 3′,5′-cyclic monophosphates cAMP and cGMP.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Daisuke Okada et al.
Biochemistry, 41(30), 9672-9679 (2002-07-24)
The effects of cGMP binding on the catalytic activity of cGMP-specific, cGMP-binding phosphodiesterase (PDE5) are unclear because cGMP interacts with both allosteric and catalytic sites specifically. We studied the effects of cGMP on the hydrolysis of a fluorescent substrate analogue
Adam K Jagielski et al.
Archives of biochemistry and biophysics, 404(2), 186-196 (2002-07-31)
The effects of extracellular purinergic agonists and their breakdown products on glucose and glutamine synthesis in rabbit kidney-cortex tubules incubated with aspartate + glycerol or alanine + glycerol + octanoate were investigated. A rapid extracellular degradation of ATP was accompanied
Riegel et al.
The Journal of experimental biology, 201 (Pt 24), 3411-3418 (1998-11-18)
External application of the 3',5'-cyclic monophosphates of inosine, cytidine, uridine and thymidine stimulated the fluid secretion rate (FSR) of Malpighian tubules isolated from Drosophila melanogaster. The evidence suggested that the cyclic nucleotides acted intracellularly in some capacity. Receptors of the
M S Shapiro et al.
Biophysical journal, 78(5), 2307-2320 (2000-04-25)
In vertebrate olfactory receptors, cAMP produced by odorants opens cyclic nucleotide-gated (CNG) channels, which allow Ca(2+) entry and depolarization of the cell. These CNG channels are composed of alpha subunits and at least two types of beta subunits that are
R C Ganassin et al.
Journal of cellular physiology, 160(3), 409-416 (1994-09-01)
The influence of inosine on DNA synthesis by Chinook salmon embryo cells (CHSE-214) was investigated because previously cell number was shown to increase from six- to thirtyfold if inosine was added to the basal medium (L-15) supplemented with either dialyzed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service