H7002
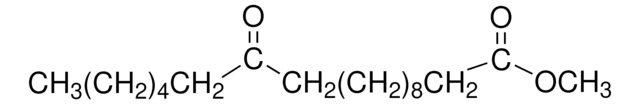
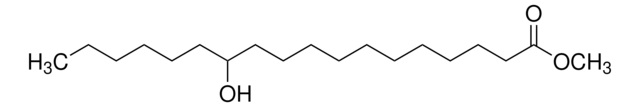
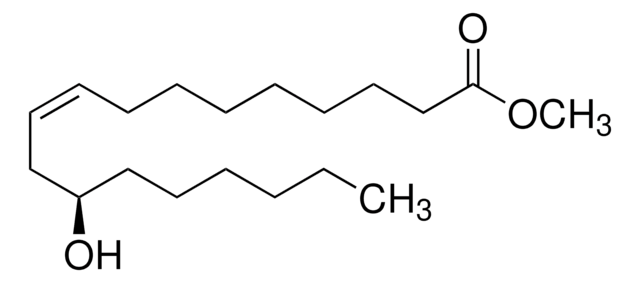
Methyl 12-hydroxystearate
≥99% (GC)
Synonym(s):
12-Hydroxystearic acid methyl ester, Methyl 12-hydroxyoctadecanoate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C19H38O3
CAS Number:
Molecular Weight:
314.50
EC Number:
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥99% (GC)
form
powder
functional group
ester
lipid type
saturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCCC(O)CCCCCCCCCCC(=O)OC
InChI
1S/C19H38O3/c1-3-4-5-12-15-18(20)16-13-10-8-6-7-9-11-14-17-19(21)22-2/h18,20H,3-17H2,1-2H3
InChI key
RVWOWEQKPMPWMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Chemical Changes of Hydroperoxy-, Epoxy-, Keto- and Hydroxy-Model Lipids under Simulated Gastric Conditions.: This study explores the stability and chemical transformations of hydroxy fatty acids, including Methyl 12-hydroxystearate, under digestive conditions, providing insight into dietary fat metabolism and its implications for nutritional sciences (Marquez-Ruiz et al., 2021).
- Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components.: This research highlights the potential environmental applications of Methyl 12-hydroxystearate, as a standard, in enhancing nitrogen cycling, important for studies on wastewater treatment and ecosystem management (Lu et al., 2014).
- Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids.: This study investigates the synthesis of derivatives of Methyl 12-hydroxystearate for potential use in food preservation and pharmaceutical applications, emphasizing its antioxidant and antifungal properties (Reddy et al., 2012).
Biochem/physiol Actions
Methyl 12-hydroxystearate is a methyl esterified fatty acid used as a reference for the identification and quantification of 12-hydroxystearic acid, a molecular organogelator, found in tissues, membranes, synthetic molecular gels and copolymers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pierre Terech et al.
The journal of physical chemistry. B, 114(35), 11409-11419 (2010-08-18)
Supercritical carbon dioxide is used to prepare aerogels of two reference molecular organogelators, 2,3-bis-n-decyloxyanthracene (DDOA) (luminescent molecule) and 12-hydroxystearic acid (HSA). Electron microscopy reveals the fibrillar morphology of the aggregates generated by the protocol. SAXS and SANS measurements show that
Allergic contact dermatitis caused by bis-diglycerylpolyacyladipate-2 (Softisan® 649) owing to its 12-hydroxystearic acid content.
Daniel W Shaw
Contact dermatitis, 65(6), 369-370 (2011-11-15)
Mark T Elsesser et al.
Langmuir : the ACS journal of surfaces and colloids, 26(23), 17989-17996 (2010-11-09)
Polymeric stabilizers are an essential ingredient for the dispersion polymerization of poly(methyl methacrylate) (PMMA) in nonpolar media. In this contribution, we focus on the synthesis of an amphipathic copolymer consisting of pendant poly(12-hydroxystearic acid) (PHS) chains grafted to an insoluble
M S Jie et al.
Lipids, 27(1), 59-64 (1992-01-01)
Methyl oleate (18:1) and linoleate (18:2) were readily transformed to the corresponding gem-dichlorocyclopropane derivatives in high yield, using triethylbenzylammonium chloride as the phase-transfer catalyst in the presence of aqueous NaOH and CHCl3. Reaction of dichlorocarbene with methyl 12-hydroxystearate furnished methyl
Allergic contact dermatitis to methyl hydroxystearate in a rubber respirator.
Emma C Benton et al.
Contact dermatitis, 67(4), 238-239 (2012-09-11)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service