G5545
Anti-β-Glucuronidase (C-Terminal) antibody produced in rabbit
~1.5 mg/mL, affinity isolated antibody, buffered aqueous solution
Synonym(s):
Anti-GUS
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
conjugate:
unconjugated
application:
WB
clone:
polyclonal
species reactivity:
plant
citations:
5
technique(s):
western blot: 1-2 μg/mL using purified GUS from E. coli
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
antigen 60 kDa
species reactivity
plant
concentration
~1.5 mg/mL
technique(s)
western blot: 1-2 μg/mL using purified GUS from E. coli
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
General description
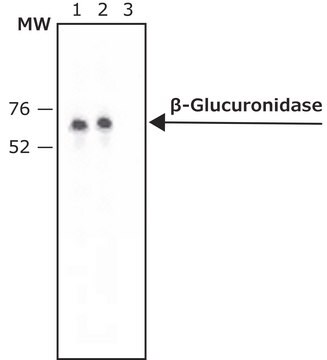
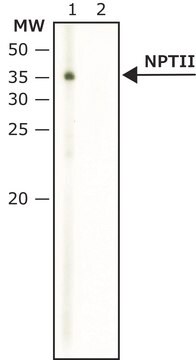
β-Glucuronidase (GUS) gene (also referred to as uidA) from Escherichia- coli, codes for a 60kDa protein.
Specificity
Anit-β-Glucuronidase (C-Terminal) recognizes bacterial GUS expressed in transgenic tobacco plants.
The antibody recognizes bacterial GUS expressed in transgenic tobacco plants.
Immunogen
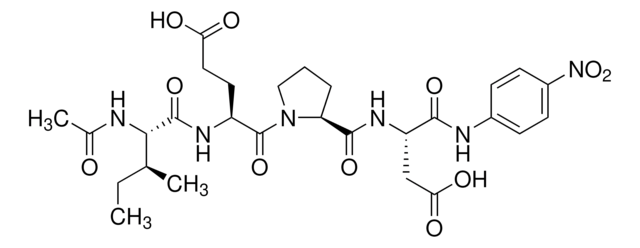
synthetic peptide corresponding to amino acids 589-603 at the C-terminus of E. coli GUS, conjugated to KLH.
Application
Detection of GUS by immunoblotting (60 kDa). Staining of the GUS band in immunoblotting is specifically inhibited by the immunizing GUS peptide (E. coli, amino acids 589-603).
Biochem/physiol Actions
β-Glucuronidase (GUS) acts as a reporter gene for plant studies. Reporter genes are widely used for studying the expression of foreign genes in transformed plant tissues. GUS is an hydrolase that catalyzes the cleavage of a variety of β-glucuronide derivatives available for colorimetric, fluorometric and histochemical assays. GUS activity is easily assayed in vitro and can withstand fixation, enabling histochemical localization in cells and tissue sections. However, one of the major limitations of the gus reporter gene system is that the histochemical GUS assay system is destructive for the plant tissue, and therefore it is not suitable for direct visual selection of transformed plants.
Physical form
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide.
Preparation Note
The antibody is affinity-purified using the immunizing peptide immobilized on agarose.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
related product
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Benjamin Dugdale et al.
The Plant cell, 25(7), 2429-2443 (2013-07-11)
In this study, we describe a novel protein production platform that provides both activation and amplification of transgene expression in planta. The In Plant Activation (INPACT) system is based on the replication machinery of tobacco yellow dwarf mastrevirus (TYDV) and
Mark D Harrison et al.
Plant biotechnology journal, 9(8), 884-896 (2011-03-02)
A major strategic goal in making ethanol from lignocellulosic biomass a cost-competitive liquid transport fuel is to reduce the cost of production of cellulolytic enzymes that hydrolyse lignocellulosic substrates to fermentable sugars. Current production systems for these enzymes, namely microbes
Sebastian N W Hoernstein et al.
Molecular & cellular proteomics : MCP, 15(6), 1808-1822 (2016-04-14)
Protein arginylation is a posttranslational modification of both N-terminal amino acids of proteins and sidechain carboxylates and can be crucial for viability and physiology in higher eukaryotes. The lack of arginylation causes severe developmental defects in moss, affects the low
Biolistic-mediated genetic transformation of cowpea (Vigna unguiculata) and stable Mendelian inheritance of transgenes
Ivo Nayche L, et al.
Plant Cell Reports, 27(9), 1475-1483 (2008)
Transgenic Plants: Gene Constructs, Vector and Transformation Method
New Visions in Plant Science (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service