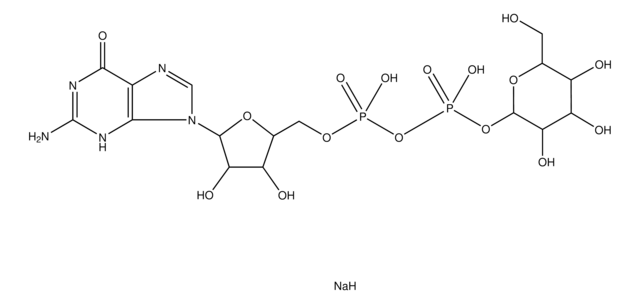
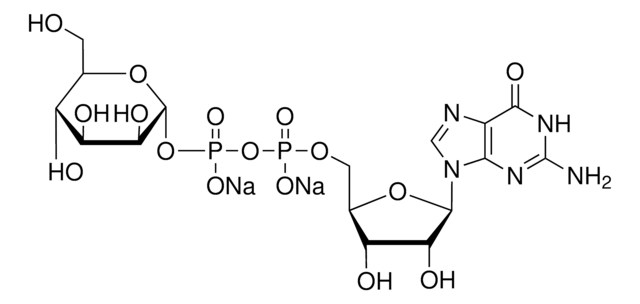
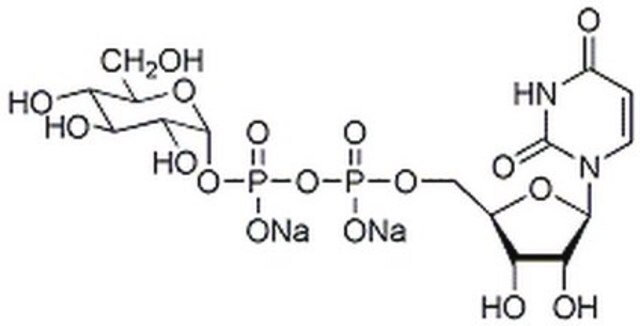
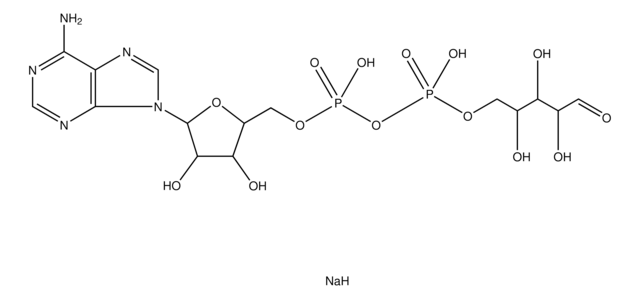
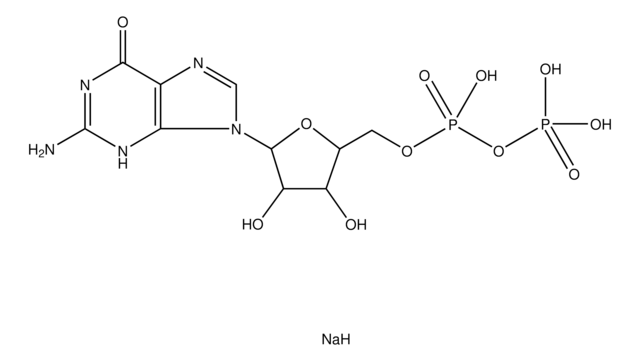
G4401
Guanosine 5′-diphospho-β-L-fucose sodium salt
≥85%, powder
Synonym(s):
6-Deoxy-β-L-galactopyranosylguanosine 5′-diphosphate, GDP-Fuc, GDP-fucose
About This Item
Recommended Products
product name
Guanosine 5′-diphospho-β-L-fucose sodium salt, ≥85%
Quality Level
Assay
≥85%
form
powder
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
CC1OC(OP(O)(=O)OP(O)(=O)OCC2OC(C(O)C2O)n3cnc4C(=O)N=C(N)Nc34)C(O)C(O)C1O
InChI
1S/C16H25N5O15P2/c1-4-7(22)9(24)11(26)15(33-4)35-38(30,31)36-37(28,29)32-2-5-8(23)10(25)14(34-5)21-3-18-6-12(21)19-16(17)20-13(6)27/h3-5,7-11,14-15,22-26H,2H2,1H3,(H,28,29)(H,30,31)(H3,17,19,20,27)
InChI key
LQEBEXMHBLQMDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Substrates
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The presence of multiple functional groups and stereocenters in complex carbohydrates makes them challenging targets for the organic chemist.
A sensitive LC-MS/MS quantitative method for the simultaneous analysis of highly polar 11 nucleotide activated sugars having similar structures and physicochemical properties using Supel™ Carbon LC column.
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service