C3888
Carboxypeptidase Y from baker′s yeast (S. cerevisiae)
lyophilized powder, ≥50 units/mg protein
Synonym(s):
Peptidyl-L-amino acid Hydrolase, Serine Carboxypeptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
grade
Proteomics Grade
Quality Level
form
lyophilized powder
specific activity
≥50 units/mg protein
mol wt
61 kDa
composition
Protein, ≥75% E1%/280
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Carboxypeptidase Y has a broad specificity and is stable in urea. Hence, this enzyme is not like other carboxypeptidases and can be used for sequence analysis. Due to its amidase action, this enzyme might be applied to the sequence analysis of peptides having amidated COOH-terminal groups such as oxytocin and vasopressin.
Biochem/physiol Actions
The glycoprotein has a molecular weight of about 61,000, has a nitrogen content of 12.74%. It is a single polypeptide chain of 442 residues with 16 residues of glucosamine in the carbohydrate moiety. Lysine is at the NH2 terminus and -Asp-Ser-Thr-Leu is the COOH-terminal sequence. The principal action of the enzyme is to remove COOH-terminal residues from polypeptide chains. It is used as a vacuolar marker enzyme for studies on protein transport and localization.
Packaging
Package size based on protein content
Unit Definition
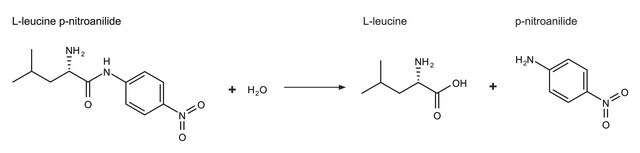
One unit will hydrolyze 1.0 μmole of N-CBZ-Phe-Ala to N-CBZ-L-phenylalanine and L-alanine per min at pH 6.75 at 25°C, based on EM/230 = 191.5.
Physical form
Lyophilized powder containing citric acid.
Analysis Note
amidase and esterase activities may be present
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Caroline A Magalhães et al.
Memorias do Instituto Oswaldo Cruz, 106(2), 146-152 (2011-05-04)
Typical and atypical enteropathogenic Escherichia coli (EPEC) are considered important bacterial causes of diarrhoea. Considering the repertoire of virulence genes, atypical EPEC (aEPEC) is a heterogeneous group, harbouring genes that are found in other diarrheagenic E. coli pathotypes, such as
H R Stennicke et al.
Biochemistry, 35(22), 7131-7141 (1996-06-04)
The activity of serine carboxypeptidases is dependent on a catalytic triad, an oxyanion hole, and a binding site equivalent to those found in the serine endopeptidases. The action of carboxypeptidase Y on substrates containing amino acids, alcohols, and amines as
L Ballou et al.
Proceedings of the National Academy of Sciences of the United States of America, 87(9), 3368-3372 (1990-05-01)
The N-linked oligosaccharides from baker's yeast carboxypeptidase Y were analyzed by 1H NMR and specific mannosidase digestion and found to be identical to those from the Saccharomyces cerevisiae mnn9 mutant bulk mannoprotein. The results support the view that the mnn
Johanna Rankenberg et al.
Experimental eye research, 210, 108704-108704 (2021-07-25)
Advanced glycation end products (AGEs) accumulate with age in human lens capsules. AGEs in lens capsules potentiate the transforming growth factor beta-2-mediated mesenchymal transition of lens epithelial cells, which suggests that they play a role in posterior capsule opacification after
Václava Bauerová et al.
Canadian journal of microbiology, 58(5), 678-681 (2012-04-17)
Vacuoles play an important role in the physiology of pathogenic Candida spp. However, information on Candida albicans vacuolar enzymes, their properties, and regulation is scarce. Expression of the genes APR1 and CPY1 encoding vacuolar aspartic protease and serine carboxypeptidase, respectively
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service