76427
Penicillin Amidase from Escherichia coli
5-10 units/mg protein
Synonym(s):
Penicillin Acylase, Penicillin Amidohydrolase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Escherichia coli
form
suspension
specific activity
5-10 units/mg protein
mol wt
Mr ~70000
technique(s)
activity assay: suitable
application(s)
diagnostic assay manufacturing
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Penicillin amidase is a periplasmic 80K heterodimer with A and B chains (209 and 566 amino acids, respectively). It is widely distributed among microorganisms, including bacteria, yeast and filamentous fungi. Among all the sources, the enzyme produced by E. coli is most well-characterized and common for industrial application.
Application
Penicillin amidase was used to study its effect in release of fatty acid and HSL (homoserine lactone) from AHLs (N -acylhomoserine lactones) in degradation of antibiotics. It was used as positive control for assaying penicillin G acylase activity in the study of functional analysis of bile salt hydrolase and penicillin acylase family members in Lactobacillus sp. Penicillin amidase may be used for synthesis of 6-aminopenicillanic acid from penicillin-G and for the industrial production of β-lactam antibiotics.
Biochem/physiol Actions
The biosynthesis of Penicillin amidase in E. coli by hydrophobic protein chromatography is an inducible reaction which is regulated by metabolized carbon source (e.g. polyols, carboxylic acid etc.). It is also influenced by catabolite repression. It catalyzes the formation of amide bonds through an acyl-enzyme intermediate.
Unit Definition
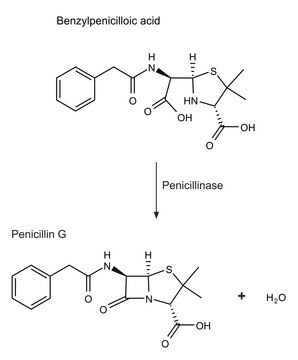
1 U corresponds to the amount of enzyme which hydrolyzes 1 μmol benzylpenicillin per minute at pH 7.6 and 37°C
Other Notes
Characterization; In enantioselective resolution; Synthesis of ampicillin and benzylpenicillin
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Penicillin Acylase in the Industrial Production of ?-Lactam Antibiotics
Bruggink A, Roos EC, Vroom ED
Organic Process Research & Development, 2(2), 128-133 (1998)
A. Guy et al.
Bioorganic & Medicinal Chemistry Letters, 3, 1041-1041 (1993)
H J Duggleby et al.
Nature, 373(6511), 264-268 (1995-01-19)
Penicillin acylase (penicillin amidohydrolase, EC 3.5.1.11) is widely distributed among microorganisms, including bacteria, yeast and filamentous fungi. It is used on an industrial scale for the production of 6-aminopenicillanic acid, the starting material for the synthesis of semi-synthetic penicillins. Its
Ampicillin and cephalexin synthesis catalysed by E. coli penicillin amidase. Yield increase due to substrate recycling
Kasche, V
Biotechnology Letters, 7, 877-882 (1985)
V Kasche et al.
Hoppe-Seyler's Zeitschrift fur physiologische Chemie, 365(12), 1435-1443 (1984-12-01)
Hydrophobic protein chromatography was used to prepare homogeneous fractions of penicillin amidase (EC 3.5.1.11) from E. coli. The apparent ratios of the rate constants for the deacylation of the acyl-penicillin amidase formed in the hydrolysis of phenylacetylglycine or D-phenylglycine methyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service