T109
L-(+)-Tartaric acid
≥99.5%
Synonym(s):
(2R,3R)-(+)-Tartaric acid, L-Threaric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
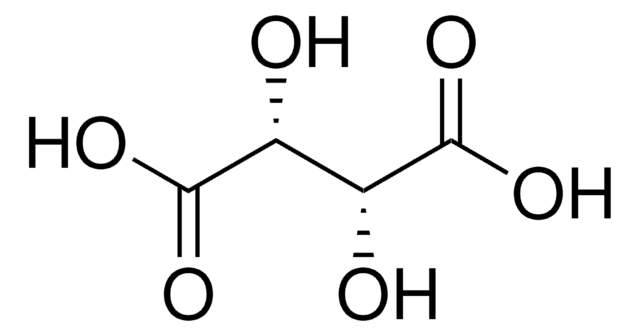
HO2CCH(OH)CH(OH)CO2H
CAS Number:
Molecular Weight:
150.09
Beilstein:
1725147
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
vapor density
5.18 (vs air)
Quality Level
Assay
≥99.5%
form
granular
powder or crystals
optical activity
[α]20/D +13.5±0.5°, c = 10% in H2O
autoignition temp.
797 °F
mp
170-172 °C (lit.)
SMILES string
O[C@H]([C@@H](O)C(O)=O)C(O)=O
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
InChI key
FEWJPZIEWOKRBE-JCYAYHJZSA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-(+)-tartaric acid is an organic compound that is commonly used as a chiral auxiliary in reactions that involve the addition of a chiral nucleophile. It is also used as a resolving agent for the separation of enantiomers from a racemic mixture. Additionally, L-(+)-tartaric acid can also be used as a building block for the synthesis of other chiral compounds.
Application
L- (+)-Tartaric acid can be used as:
- A co-former for the synthesis of etravirine co-crystals.
- A mobile phase additive in thin-layer chromatography.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amalia Papadaki et al.
Bio-protocol, 9(19), e3384-e3384 (2019-10-05)
Acid ecto-phosphatases are enzymes that hydrolyze phosphomonoesters in the acidic pH range with their active sites facing the extacellular medium. Their activities can be measured in living cells. In bacteria and protozoan pathogens, acid ecto-phosphatases have been associated with the
Preparation of organotrifluoroborate salts: precipitation-driven equilibrium under non-etching conditions.
Alastair J J Lennox et al.
Angewandte Chemie (International ed. in English), 51(37), 9385-9388 (2012-08-21)
Yanfang Feng et al.
Bioresource technology, 125, 138-144 (2012-10-03)
The aim of this study was to develop a promising and competitive bioadsorbent with the abundant of source, low price and environmentally friendly characters to remove cationic dye from wastewater. The swede rape straw (Brassica napus L.) modified by tartaric
Andrea Bencini et al.
Chemical communications (Cambridge, England), 48(84), 10428-10430 (2012-09-18)
A chiral ditopic polyammonium receptor featuring two [9]aneN(3) moieties separated by a (S)-BINOL linker is able to selectively bind and sense in water (S,S)-tartaric acid over its (R,R)/meso forms.
Mrinal Kanti Bain et al.
Carbohydrate polymers, 91(2), 529-536 (2012-11-06)
Gelation temperature of MC was reduced from 59°C to 54°C with the addition of 10% PEG. Sodium tartrate (NaT) and sodium citrate (NaC) were added to the MC-PEG solution to further reduce the gelation temperature close to physiological temperature. Different
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service