T0254
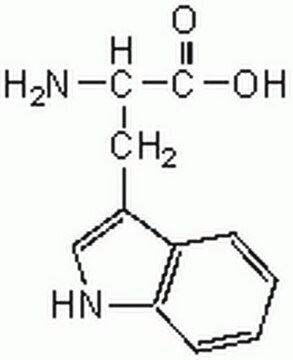
L-Tryptophan
≥98% (HPLC)
Synonym(s):
(S)-2-Amino-3-(3-indolyl)propionic acid, L-α-Amino-3-indolepropionic acid
About This Item
Recommended Products
product name
L-Tryptophan, reagent grade, ≥98% (HPLC)
grade
reagent grade
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to off-white
mp
280-285 °C (dec.) (lit.)
solubility
0.5 M HCl: 50 mg/mL
application(s)
cell analysis
SMILES string
N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
InChI key
QIVBCDIJIAJPQS-VIFPVBQESA-N
Gene Information
human ... TDO2(6999) , TPH1(7166) , TPH2(121278)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
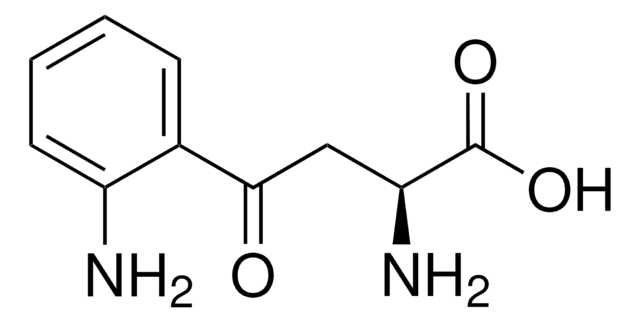
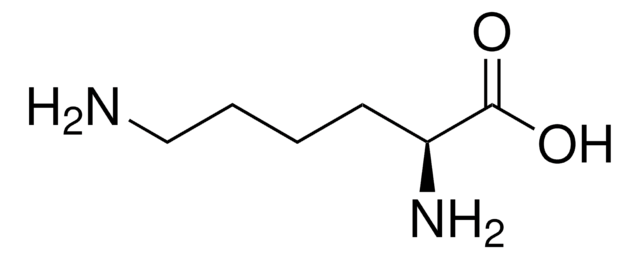
Customers Also Viewed
Articles
Serotonin (5-hydroxytryptamine) is principally found stored in three main cell types - i) serotonergic neurons in the CNS and in the intestinal myenteric plexus, ii) enterochromaffin cells in the mucosa of the gastrointestinal tract, and iii) in blood platelets. Metabolism of serotonin is carried out primarily by the outer mitochondrial membrane enzyme monoamine oxidase (MAO), which occurs as two molecular subtypes called MAO-A and MAO-B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service