M9140
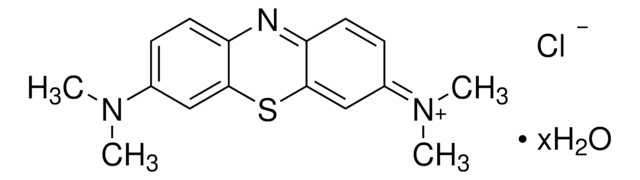
Methylene blue
Dye content, ≥82%, certified by the Biological Stain Commission, powder
Synonym(s):
Basic Blue 9, 3,7-bis(Dimethylamino)phenazathionium chloride, Tetramethylthionine chloride
About This Item
Recommended Products
Product Name
Methylene blue, certified by the BSC, certified by the Biological Stain Commission
grade
certified by the Biological Stain Commission
Quality Level
Agency
certified by the BSC
form
powder
composition
Dye content, ≥82%
technique(s)
microbe id | staining: suitable
color
dark green
solubility
H2O: 4mg/4ml
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
CN(C)C1=CC=C2C(SC(C3=N2)=CC(C=C3)=[N+](C)C)=C1.[H]O[H].[Cl-]
InChI
1S/C16H18N3S.ClH.H2O/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13;;/h5-10H,1-4H3;1H;1H2/q+1;;/p-1
InChI key
WQVSELLRAGBDLX-UHFFFAOYSA-M
Gene Information
human ... CYP1A2(1544) , GSR(2936)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to stain vaginal cells
- for the preparation of protein aqueous ink
- to evaluate cell survival by staining
Biochem/physiol Actions
Suitability
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Serotonin (5-hydroxytryptamine) is principally found stored in three main cell types - i) serotonergic neurons in the CNS and in the intestinal myenteric plexus, ii) enterochromaffin cells in the mucosa of the gastrointestinal tract, and iii) in blood platelets. Metabolism of serotonin is carried out primarily by the outer mitochondrial membrane enzyme monoamine oxidase (MAO), which occurs as two molecular subtypes called MAO-A and MAO-B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service