195758
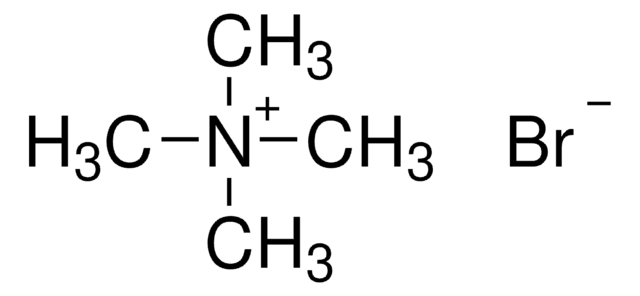
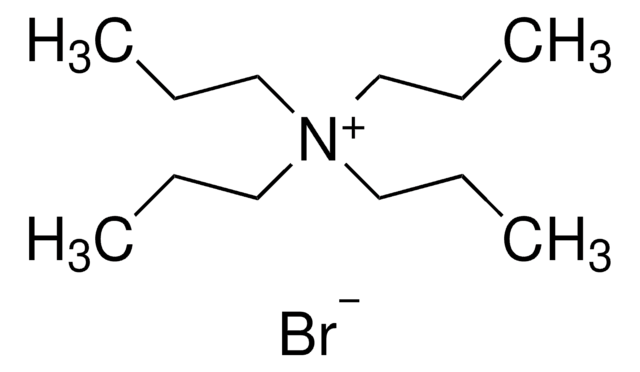
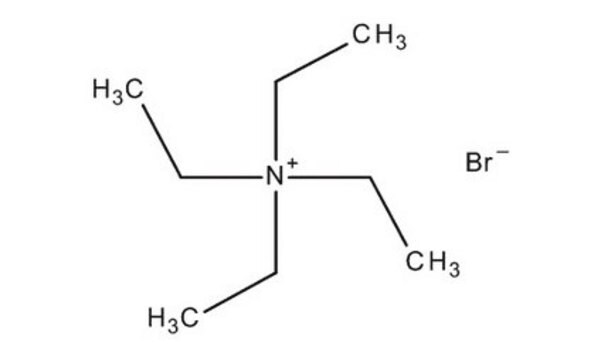
Tetramethylammonium bromide
98%
Synonym(s):
TMAB
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)4N(Br)
CAS Number:
Molecular Weight:
154.05
Beilstein:
3620955
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
98%
form
solid
mp
>300 °C (lit.)
functional group
amine
SMILES string
[Br-].C[N+](C)(C)C
InChI
1S/C4H12N.BrH/c1-5(2,3)4;/h1-4H3;1H/q+1;/p-1
InChI key
DDFYFBUWEBINLX-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetrabutylammonium bromide is a quaternary ammonium compound that is widely used as a phase transfer catalyst. Absolute viscosities of its aqueous solutions have been measured at various temperatures (20,25 and 30°C). Various physical properties (activity coefficient up to saturation, conductance, density and solubility in water) of TMABr have been evaluated.
Tetramethylammonium bromide (TMABr) is a quaternary ammonium salt that is widely used as a phase transfer catalyst. Absolute viscosities of its aqueous solutions have been measured at various temperatures (20,25 and 30°C). Various physical properties (activity coefficient up to saturation, conductance, density and solubility in water) of TMABr have been evaluated. Its impact on the first order rate constant values for the base hydrolysis of the following Fe(II) chelates has been investigated:
- bis(naphthylidene alanate) (nali)
- bis(naphthylidene phenylalanate) (nphali)
- bis(naphthylidene aspartate) (nasi)
- (naphthylidene histidinate) (nhi)
- bis(naphthylidenearginate) (nari)
Application
Tetrabutylammonium bromide may be used in the molten state in the following processes:
- Synthesis of (2S)-5-(3-phenyl-2-phthalimidylpropanoylamino)isophthalic acid.
- Synthesis of alkyl-substituted pyrroles in the absence of catalyst and organic solvent.
- Synthesis of dithioacetals from acetals by transthioacetalisation in a solvent free environment.
- Synthesis of polyamides (PAs) by the polymerization of terephthalic acid and diisocyanates.
- Catalyze the addition of thiols to conjugated alkenes.
- Dehydrochlorination of poly(vinyl chloride).
Tetramethylammonium bromide may be used as a template in the synthesis of small colloidal zeolite Y nanocrystals.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dehydrochlorination of poly (vinyl chloride) by aqueous sodium hydroxide solution under two-phase conditions.
Kise H.
Journal of Polymer Science, 20(11), 3189-3197 (1982)
Some physical properties of aqueous solutions of tetramethylammonium bromide and tetramethylammonium iodide
Levien BJ.
Australian Journal of Chemistry, 18(8), 1161-1170 (1965)
Controlling size and yield of zeolite Y nanocrystals using tetramethylammonium bromide.
Holmberg BA, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 59(1), 13-28 (2003)
Viscosity of aqueous solutions. III. Tetramethylammonium bromide and the role of the tetraalkylammonium ions.
Nightingale Jr ER.
The Journal of Physical Chemistry, 66(5), 894-897 (1962)
Catalysis by ionic liquids: solvent-free efficient transthioacetalisation of acetals by molten tetrabutylammonium bromide.
Ranu BC, et al.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1520-1522 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service