MXRDP2DT00
Milliflex® Rapid 2.0 Detection Tower with no power supply
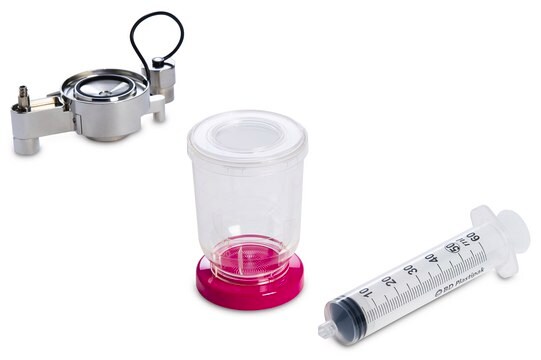
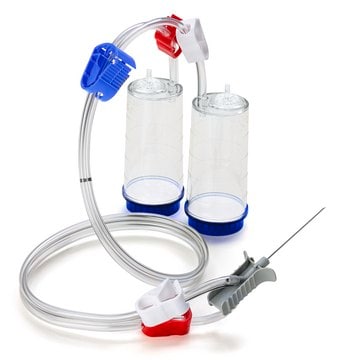
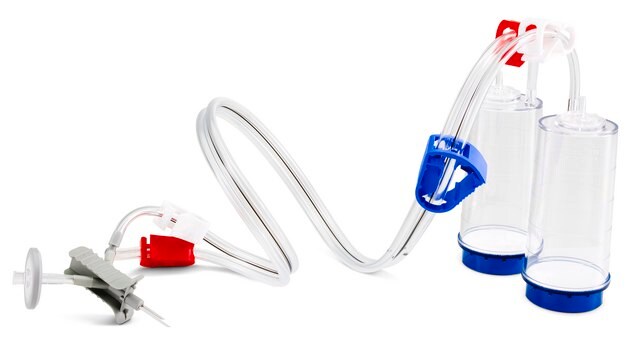
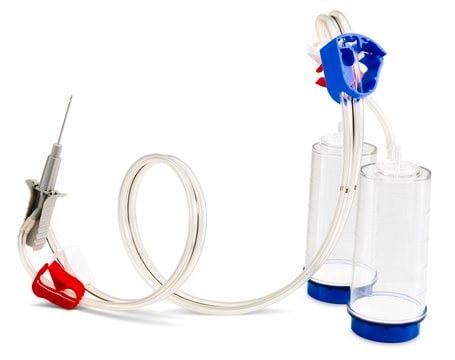
The Milliflex® Rapid2.0 detection tower is suitable for bioburden and sterility testing, for use with Milliflex® Rapid System 2.0, suitable for sterility testing
Synonym(s):
response and resolution of microbial contamination
About This Item
Recommended Products
material
acrylonitrile-butadiene-styrene (ABS) (rear panel)
acrylonitrile-butadiene-styrene (ABS) housing
aluminum inner door
packaging
pkg of 1 unit
manufacturer/tradename
Milliflex®
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
parameter
15-32 °C temperature
technique(s)
Rapid Sterility Testing: suitable
W × depth × H
297 mm × 126 mm × 348 mm
weight
5.2 kg
input
sample type pharmaceutical(s)
application(s)
bioburden testing
pharmaceutical
sterility testing
compatibility
for use with Milliflex® Rapid System 2.0
greener alternative category
shipped in
ambient
General description
Application
Features and Benefits
- Consistently fast and reproducible results
- Plug & Play concept
- Data integrity thanks to 21 CFR Part 11 compliant software
- Easy handling and validation
Other Notes
Instrument Power input :24 V DC 50 W. max per equipment connected
Legal Information
related product
required but not provided
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Use Milliflex® Rapid System to detect cell-based immunotherapeutics for microbial contaminants. This automated ATP-based system supports rapid detection, imaging, and quantification of viable microorganisms.
Milliflex® Rapid System 2.0, an automated solution for rapid microbial detection supports QC labs to substantially reduce the time-to-result of sterility, bioburden, in-process, and product release testing. Thanks to the ATP bioluminescence technology, the system delivers 4 times faster results than traditional methods.
Related Content
The automated Milliflex® Rapid system can get you bioburden testing and sterility testing results days earlier. Its proven technology rapidly and accurately detects microorganisms in sterility, bioburden, in-process and product release testing.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service