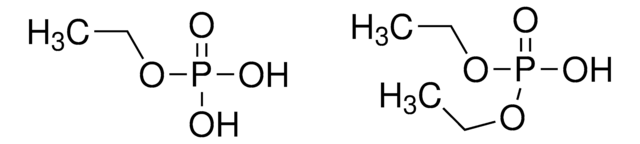8.07471
Polyphosphoric acid
for synthesis
Synonym(s):
Polyphosphoric acid, PPA
About This Item
Recommended Products
vapor pressure
2 hPa ( 20 °C)
Quality Level
Assay
83-87% P2O5 basis (acidimetric)
form
viscous liquid
bp
530 °C/1013 hPa
mp
-20 °C
density
2.06 g/cm3 at 20 °C
storage temp.
2-30°C
SMILES string
[P](=O)(O)(O)O.[p]21[o][p]([o][o]2)[o][o]1
InChI
1S/O5P2.H3O4P/c1-2-7-4-3-6(1)5-7;1-5(2,3)4/h;(H3,1,2,3,4)
InChI key
FVYIVXLIMHNYTD-UHFFFAOYSA-N
General description
Application
- In acylation and alkylation reactions.
- To synthesize cyclic aromatic compounds via cyclization of aromatic acids, esters, ketones, aldehydes, acetals, alcohols, and alkenes.
- In Bischler−Napieralski reaction to construct isoquinoline ring system from phenethylformamides.
- To prepare substituted indoles at position-2 from hydrazones via Fisher indole synthesis.
- For the conversion of oximes into amides via Beckmann rearrangement.
PPA can also be used as a catalyst to synthesize:
- Aniline derivatives by Lossen rearrangement of aromatic carboxylic acids with nitromethane.
- Amides by hydrolysis nitriles.
- Dimethyl carbonate (DMC) from urea and methanol.
- Benzofurans by cyclization of α-phenoxy ketones.
PPA is also used with other reagents for various organic transformations. For example:
- PPA/Ethanethiol mixture can be used to selectively open one epoxide in bis-epoxy steroid.
- PPA/POCl3 catalytic mixture used to convert tertiary alcohols into corresponding chlorides.
- PPA/acetic acid is used to synthesize nonenolizable β-diketones.
- PPA/potassium iodide mixture used to convert primary methyl ether to a primary alkyl iodide.
- PPA/nitric acid catalytic combination used for nitration reactions which is less hazardous than Ac2O/HNO3 mixtures.
Analysis Note
Due to its specific melting range the product may be solid, liquid, a solidified melt or a supercooled melt.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service