Recommended Products
Quality Level
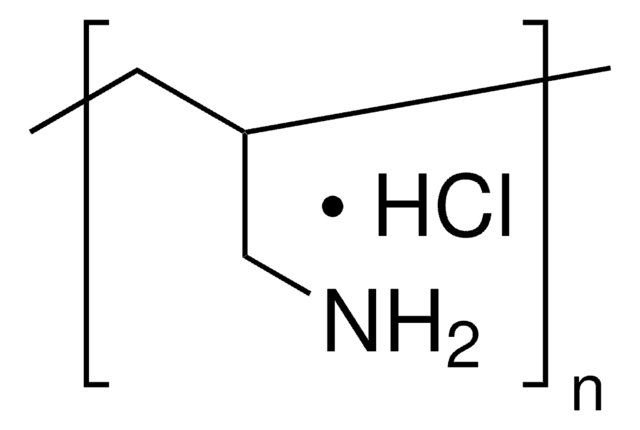
form
liquid
pH
7.8 ( in H2O, ready-to-use solution)
density
1.015 g/cm3
storage temp.
15-25°C
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Multi column chromatography has the benefit of using less resin, increasing productivity, and enabling continuous processing. Learn more in this technical article.
Influenza vaccines are commonly made using egg-based and cell-based manufacturing strategies. Find step-by-step information on the manufacturing process for each method.
A custom-designed cost model is used to explore the economics of vaccine manufacturing across several different modalities including mRNA. The model enables greater process understanding, simulates bottlenecks, and helps to optimize production efficiency.
Learn more one the attenuated viral vaccines manufacturing process: cell culture, clarification, nuclease treatment, chromatography, and sterile filtration.
Related Content
Flocculants, like pDADMAC, remove cell debris during downstream monoclonal antibody production, but their adoption has been difficult due to perceived regulatory concerns.
Learn more on mAb downstream processing, more specifically clarification and the relevant associated products.
This technical article breaks down the adenovirus vaccine manufacturing process and provides a case study on developing an accelerated and cost-effective single-use adenoviral vector vaccine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service