890700P
Avanti
12:0 EPC (Cl Salt)
Avanti Polar Lipids 890700P, powder
Synonym(s):
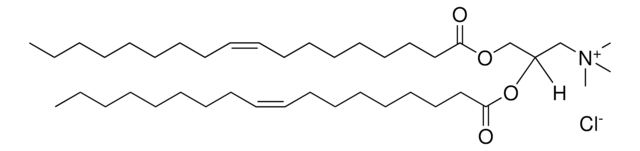
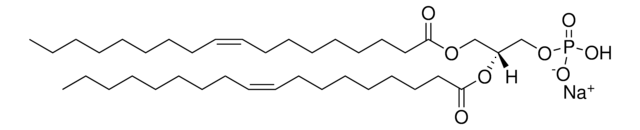
1,2-dilauroyl-sn-glycero-3-ethylphosphocholine (chloride salt)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C34H69NO8PCl
CAS Number:
Molecular Weight:
686.34
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 10 mg (890700P-10mg)
manufacturer/tradename
Avanti Polar Lipids 890700P
lipid type
cationic lipids
transfection
shipped in
dry ice
storage temp.
−20°C
SMILES string
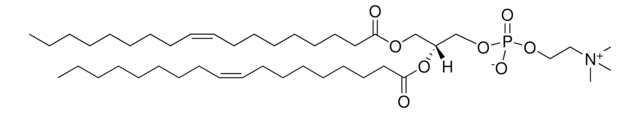
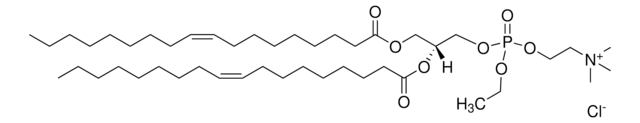
O=P(OCC[N+](C)(C)C)(OC[C@]([H])(OC(CCCCCCCCCCC)=O)COC(CCCCCCCCCCC)=O)OCC.[Cl-]
General description
1,2-dilauroyl-sn-glycero-3-ethylphosphocholine (12:0 EPC) is a cationic phospholipid. 12:0 EPC is a short-chain analog of dioleoyl ethylphosphatidylcholine. O-alkyl phosphatidylcholines constitute the first chemically stable triesters of biological lipid structures and the first cationic derivatives of phospholipids consisting entirely of biological metabolites linked with ester bonds. The lipid has low toxicity and is biodegradable.
Application
1,2-dilauroyl-sn-glycero-3-ethylphosphocholine (12:0 EPC (Cl Salt)) is suitable for use in lipoplex preparation and to study the lipoplex transfection activity. It might also be used in lipid mixtures to study its cubic phases of formation, stability and phase transitions.
Biochem/physiol Actions
1,2-dilauroyl-sn-glycero-3-ethylphosphocholine (12:0 EPC) is preferred for transfection of cultured primary endothelial cells. 12:0 EPC forms lamellar lipoplexes possessing high to superior transfection activity. Chemically synthesized cationic lipids are applicable as non-viral gene carriers. Variation in the hydrocarbon chain of phosphatidylcholine (PC) derivatives affects transfection efficiency.
Packaging
5 mL Clear Glass Sealed Ampule (890700P-10mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rumiana Koynova et al.
The journal of physical chemistry. B, 111(27), 7786-7795 (2007-06-19)
Some mixtures of two cationic lipids including phospholipid compounds (O-ethylphosphatidylcholines) as well as common, commercially available cationic lipids, such as dimethylammonium bromides and trimethylammonium propanes, deliver therapeutic DNA considerably more efficiently than do the separate molecules. In an effort to
Boris G Tenchov et al.
Biochimica et biophysica acta, 1778(10), 2405-2412 (2008-08-30)
Synthetic cationic lipids can be used as DNA carriers and are regarded to be the most promising non-viral gene carriers. For this investigation, six novel phosphatidylcholine (PC) cationic derivatives with various hydrophobic moieties were synthesized and their transfection efficiencies for
Rumiana Koynova et al.
Biochimica et biophysica acta, 1768(2), 375-386 (2006-12-13)
Lipoplexes containing a mixture of cationic phospholipids dioleoylethylphosphatidylcholine (EDOPC) and dilauroylethylphosphatidylcholine (EDLPC) are known to be far more efficient agents in transfection of cultured primary endothelial cells than are lipoplexes containing either lipid alone. The large magnitude of the synergy
Li Wang et al.
Molecular pharmaceutics, 4(4), 615-623 (2007-04-06)
To date, the primary approach to improving the transfection properties of cationic lipids has been the synthesis of new kinds of cationic amphipaths. Recently, however, it was found that combining two cationic lipid derivatives having the same head group but
Rumiana Koynova et al.
Molecular pharmaceutics, 6(3), 951-958 (2009-04-04)
Synthetic cationic lipids are widely used components of nonviral gene carriers, and the factors regulating their transfection efficiency are the subject of considerable interest. In view of the important role that electrostatic interactions with the polyanionic nucleic acids play in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service