H43407
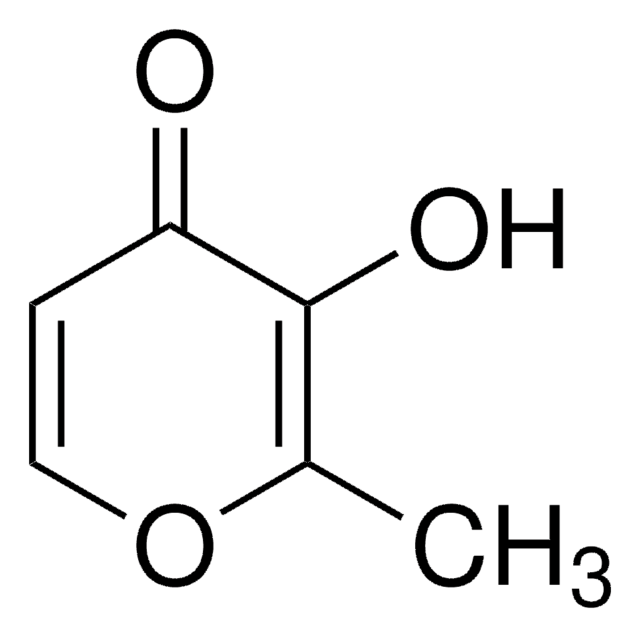
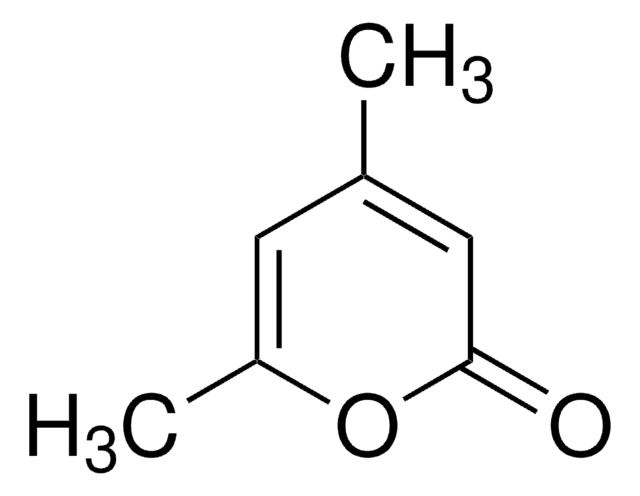
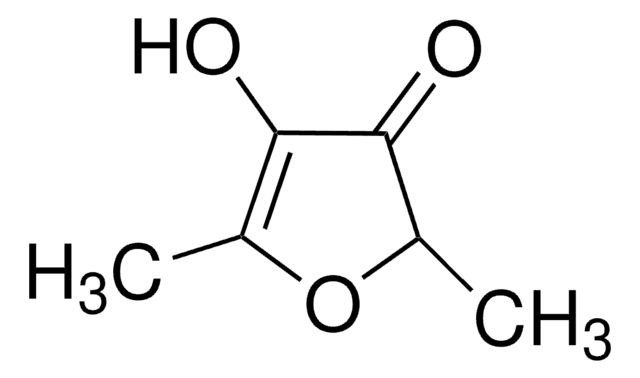
3-Hydroxy-2-methyl-4-pyrone
99%
Synonym(s):
3-Hydroxy-2-methyl-4H-pyran-4-one, Larixinic acid, Maltol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H6O3
CAS Number:
Molecular Weight:
126.11
Beilstein:
112169
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
autoignition temp.
1364 °F
expl. lim.
25 %
mp
160-164 °C (lit.)
SMILES string
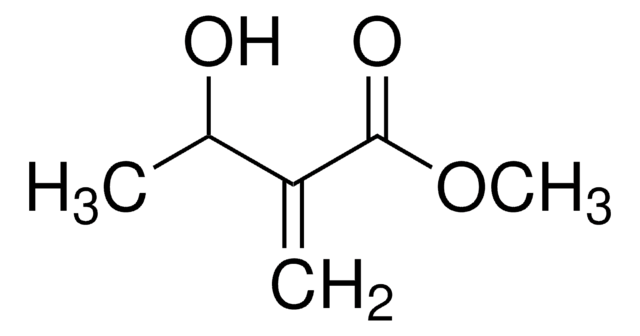
CC1=C(O)C(=O)C=CO1
InChI
1S/C6H6O3/c1-4-6(8)5(7)2-3-9-4/h2-3,8H,1H3
InChI key
XPCTZQVDEJYUGT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anwar Anwar-Mohamed et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(4), 685-690 (2007-02-24)
Maltol is used extensively as a flavor-enhancing agent, food preservative, antioxidant, and also in cosmetic and pharmaceutical formulations. However, a number of studies have shown that maltol may induce carcinogenicity and toxicity but the mechanisms involved remain unknown. Therefore, we
Tamás Jakusch et al.
Dalton transactions (Cambridge, England : 2003), (13)(13), 2428-2437 (2009-03-18)
The interactions of various insulin mimetic oxovanadium(IV) compounds with serum proteins were studied in model systems and in ex vivo samples. For the modeling study, an earlier in situ method was extended and applied to the formation of ternary complexes
Sílvia Chaves et al.
Journal of inorganic biochemistry, 114, 38-46 (2012-06-13)
The O,S-donor analogues of maltol and deferiprone (DMHP), respectively, thiomaltol and DMHTP, have been investigated in solution for their iron-complexation ability, as well as their electrochemical behaviors, in the presence and absence of iron, aimed at the rationalization of their
Christoph J Jocher et al.
Inorganic chemistry, 47(18), 7951-7953 (2008-08-19)
The synthesis, characterization, and photophysical properties are reported for several Ln(III) complexes of a tetradentate chelate, 5LIO-MAM, derived from the common flavor enhancer "maltol". Eu(III), Yb(III), and Nd(III) form stable ML2 complexes in aqueous solution that emit in the red
Stefano Amatori et al.
The Journal of organic chemistry, 77(5), 2207-2218 (2012-02-03)
The N,N'-bis[(3-hydroxy-4-pyron-2-yl)methyl]-N,N'-dimethylethylendiamine (malten) and 4,10-bis[(3-hydroxy-4-pyron-2-yl)methyl]-1,7-dimethyl-1,4,7,10-tetraazacyclododecane (maltonis) were synthesized and characterized. The acid-base behavior, structural characterizations, and biochemical studies in aqueous solution were reported. Each compound contains two 3-hydroxy-2-methyl-4-pyrone units (maltol) symmetrically spaced by a polyamine fragment, the 1,4-dimethylethylendiamine (malten), or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service