D87589
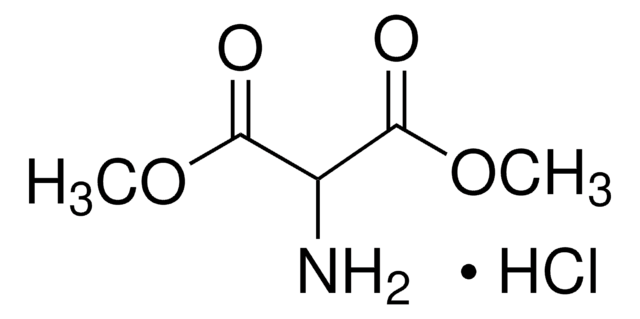
Diethyl aminomalonate hydrochloride
98%
Synonym(s):
Aminomalonic acid diethyl ester hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2(COOC2H5)2 · HCl
CAS Number:
Molecular Weight:
211.64
Beilstein:
3568037
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
165-170 °C (dec.) (lit.)
SMILES string
Cl.CCOC(=O)C(N)C(=O)OCC
InChI
1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1H
InChI key
GLFVNTDRBTZJIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C M Metzler et al.
Biochemistry, 27(13), 4923-4933 (1988-06-28)
To establish the state of protonation of quinonoid species formed nonenzymically from pyridoxal phosphate (PLP) and diethyl aminomalonate, we have studied absorption spectra of the rapidly established steady-state mixture of species. We have evaluated the formation constant and the spectrum
Seiichi Ohta et al.
Molecular pharmaceutics, 14(9), 3105-3113 (2017-08-15)
Intraperitoneal administration of chemotherapeutics is expected for the treatment of peritoneally disseminated gastric cancer because of poor migration of the drugs from the systemic circulation to the peritoneal cavity. In this study, for intraperitoneal delivery of cisplatin (CDDP), we developed
Reaction control in the organocatalytic asymmetric one-pot, three-component reaction of aldehydes, diethyl alpha-aminomalonate and nitroalkenes: toward diversity-oriented synthesis.
Yan-Kai Liu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(32), 9873-9877 (2008-10-04)
F Hughes et al.
Organic letters, 3(18), 2911-2914 (2001-09-01)
[reaction: see text]. Nitrogen-containing tethered diacids, easily prepared by reductive alkylation of diethyl aminomalonate or ethyl cyanoglycinate, undergo double Michael reactions with 3-butyn-2-one to give highly functionalized and substituted piperidines (pipecolic acid derivatives) with surprisingly high stereoselectivity. The heterocyclic double
Charles M Blazey et al.
The Journal of organic chemistry, 67(1), 298-300 (2002-01-05)
The azomethine ylide derived from the condensation of diethyl aminomalonate with paraformaldehyde undergoes 1,3-dipolar cycloadditions with acrylate and propiolate derivatives. Contrary to a previous report, these reactions yield mixtures of regioisomers generally favoring the 2,2,3-trisubstituted product. However, the relative quantity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service