C27115
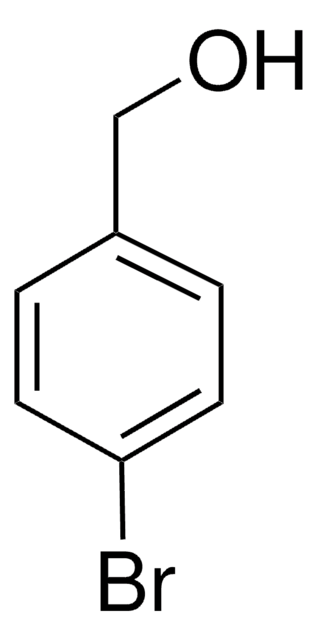
4-Chlorobenzyl alcohol
99%
Synonym(s):
1-Chloro-4-(hydroxymethyl)benzene, 4-Chlorobenzenemethanol, 4-Chlorophenylmethanol, p-Chlorobenzyl alcohol, p-Chlorotoluol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4CH2OH
CAS Number:
Molecular Weight:
142.58
Beilstein:
636502
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
bp
234 °C (lit.)
mp
68-71 °C (lit.)
SMILES string
OCc1ccc(Cl)cc1
InChI
1S/C7H7ClO/c8-7-3-1-6(5-9)2-4-7/h1-4,9H,5H2
InChI key
PTHGDVCPCZKZKR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent for carboxyl group protection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mario Silva et al.
Journal of chromatography. A, 1629, 461508-461508 (2020-08-29)
In the present document, we report the development of an analytical method consisting of a sequential direct-immersion/headspace solid-phase microextraction (DI-HS-SPME) followed by gas-phase chromatography and tandem mass spectrometry (GC-MS/MS) for simultaneous analysis of 4-chlorobenzyl alcohol, 2,6-dichlorobenzyl alcohol, 4-methoxybenzyl alcohol, 3,4-dimethoxybenzyl
Kenji Matsuno et al.
Bioorganic & medicinal chemistry letters, 20(17), 5126-5129 (2010-08-07)
S-benzylisothiourea 3a was discovered by its ability to inhibit indoleamine-2,3-dioxygenase (IDO) in our screening program. Subsequent optimization of the initial hit 3a lead to the identification of sub-muM inhibitors 3r and 10h, both of which suppressed kynurenine production in A431
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service