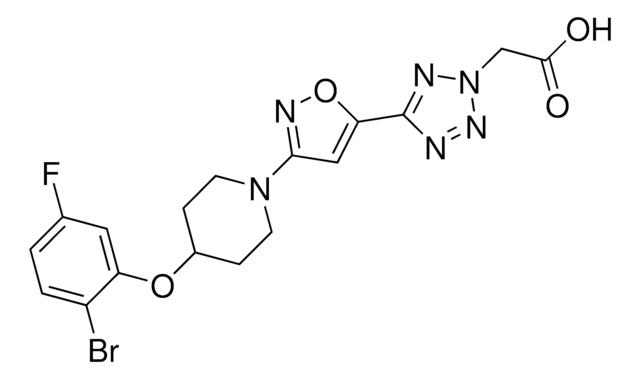
901782
tert-Butyl (R)-3-(2-acetamidopropan-2-yl)-6-chloro-5-methyl-2,3-dihydrospiro[indene-1,4′-piperidine]-1′-carboxylate
Synonym(s):
Aryl Halide Chemistry Informer Library Compound X16
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C24H35ClN2O3
CAS Number:
Molecular Weight:
435.00
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
solid
mp
210-215 °C
Application
This product is Informer compound X16 of the Aryl Halide Chemistry Informer Library developed by chemists at Merck & Co., Inc., Kenilworth, NJ, U.S., which contains 18 drug-like molecules representative of those encountered in complex synthesis. By screening a new reaction against the Informer Library, chemists can directly compare and analyse a reaction′s successes and shortcomings among different methods and various research teams. It may also be used to facilitate deeper method development for performance or utility.
Caution
Not Fully Tested.
Other Notes
1.Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods, Chem. Sci., 2016, 7, 2604-2613
2.Synthesis of Complex Druglike Molecules by the Use of Highly Functionalized Bench-Stable Organozinc Reagents
3.Synthesis of a Tertiary Carbinamide via a Novel Rh-Catalyzed Asymmetric Hydrogenation
2.Synthesis of Complex Druglike Molecules by the Use of Highly Functionalized Bench-Stable Organozinc Reagents
3.Synthesis of a Tertiary Carbinamide via a Novel Rh-Catalyzed Asymmetric Hydrogenation
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
John Limanto et al.
The Journal of organic chemistry, 73(4), 1639-1642 (2008-01-12)
Asymmetric hydrogenation of allylic dimethylcarbinamide 2 with 1 mol % of cationic Rh(I)-Josiphos complex in THF under 500 psi of H2 generated the corresponding tertiary carbinamide 1 in 98.5% assay yield and a 94:6 enantiomeric ratio. Upon crystallization, the product
Thomas J Greshock et al.
Angewandte Chemie (International ed. in English), 55(44), 13714-13718 (2016-10-22)
The reactivity of a representative set of 17 organozinc pivalates with 18 polyfunctional druglike electrophiles (informers) in Negishi cross-coupling reactions was evaluated by high-throughput experimentation protocols. The high-fidelity scaleup of successful reactions in parallel enabled the isolation of sufficient material
Peter S Kutchukian et al.
Chemical science, 7(4), 2604-2613 (2016-04-21)
Major new advances in synthetic chemistry methods are typically reported using simple, non-standardized reaction substrates, and reaction failures are rarely documented. This makes the evaluation and choice of a synthetic method difficult. We report a standardized complex molecule diagnostic approach
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service