696668
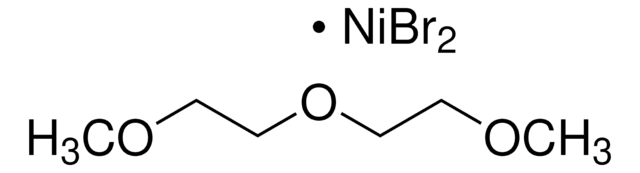
Nickel(II) chloride ethylene glycol dimethyl ether complex
98%
Synonym(s):
Dichloro(dimethoxyethane)nickel, NiCl2 glyme
About This Item
Recommended Products
Assay
98%
form
powder
reaction suitability
core: nickel
reagent type: catalyst
mp
>300 °C
SMILES string
Cl[Ni]Cl.COCCOC
InChI
1S/C4H10O2.2ClH.Ni/c1-5-3-4-6-2;;;/h3-4H2,1-2H3;2*1H;/q;;;+2/p-2
InChI key
OCMNCWNTDDVHFK-UHFFFAOYSA-L
Application
- As a catalyst for the borylation of racemic benzylic chloride to synthesize enantioenriched benzylic boronic esters.
- As a promoter for the trifluoromethylation of alkyl iodides to synthesize broad range of alkyl-CF3 compounds.
- For the synthesis of nickel bis(benzimidazol-2-ylidene) pincer complexes which can be used for electrocatalytic reduction of CO2 to CO.
- As a Lewis acid catalyst for C-acylation β-ketoesters through photoactivation by visible light.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Carc. 1B - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nickel transition metal and its complexes can be used as a catalyst in many synthetic transformations, like oxidative addition, C-H activation, reductive elimination, oxidative cyclization, oligomerization, and in cross-coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service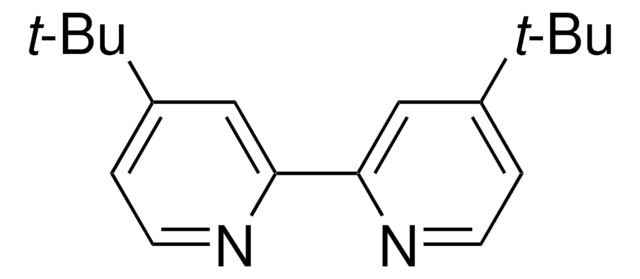
![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)







![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)

![[(TEEDA)Ni(o-tolyl)Cl] ≥95%](/deepweb/assets/sigmaaldrich/product/structures/156/227/a6ce708d-c671-4ca6-98ba-ef780504ca58/640/a6ce708d-c671-4ca6-98ba-ef780504ca58.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)