All Photos(1)
About This Item
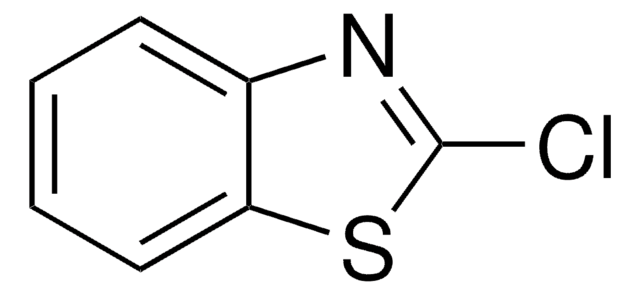
Empirical Formula (Hill Notation):
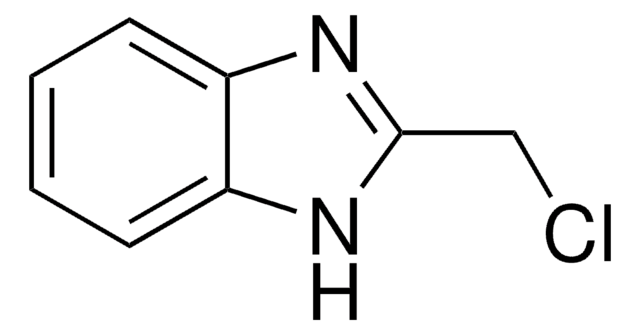
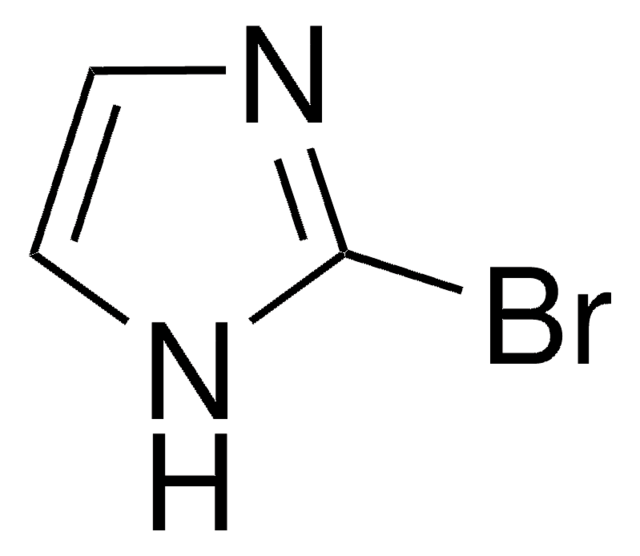
C7H5ClN2
CAS Number:
Molecular Weight:
152.58
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
207-211 °C (lit.)
functional group
chloro
SMILES string
Clc1nc2ccccc2[nH]1
InChI
1S/C7H5ClN2/c8-7-9-5-3-1-2-4-6(5)10-7/h1-4H,(H,9,10)
InChI key
AYPSHJCKSDNETA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chlorobenzimidazole can be synthesized by reacting benzimidazole-2-one with phosphoryl chloride in the presence of phenol.
Application
2-Chlorobenzimidazole can be used to synthesize the following:
- 1-methyl-2-chlorobenzimidazole via reaction with dimethyl sulfate
- 1-ethyl-2-chlorobenzimidazole via reaction with diethyl sulfate
- 1-benzyl-2-chlorobenzimidazole via reaction with benzyl chloride
- 2-chloro-4,5,6,7-tetrabromobenzimidazole via bromination with bromine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
?A facile and green synthesis of N-substituted-2-chlorobenzimidazoles?
Rao S.S, et al.
Der Pharma Chemica, 5(06), 69- 72 (2013)
?Polyhalogenobenzimidazoles: Synthesis and Their InhibitoryActivity against Casein Kinases?
Andrzejewska M, et al.
Bioorganic & Medicinal Chemistry, 11(18), 3997-4002 (2003)
?Discovery of pyrimidine benzimidazoles as Src-family selective Lck inhibitors. Part II?
Zhang G, et al.
Bioorganic & Medicinal Chemistry, 19(23), 6691-6695 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service