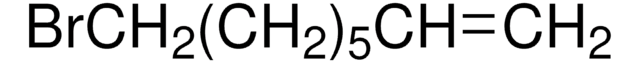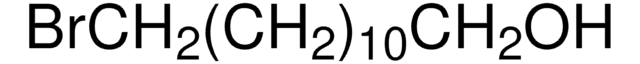All Photos(1)
About This Item
Linear Formula:
Br(CH2)8CH=CH2
CAS Number:
Molecular Weight:
219.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.4660 (lit.)
bp
50 °C/0.3 mmHg (lit.)
density
1.092 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCC=C
InChI
1S/C10H19Br/c1-2-3-4-5-6-7-8-9-10-11/h2H,1,3-10H2
InChI key
JVVPJOMYWVYPOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
10-Bromo-1-decene can be synthesized by reacting 9-decen-1-ol with PBr3. The product undergoes reduction reaction with 2-propylbenzo[d][1,3,2]dioxaborole (PBD) and Bu3SnH under room temperature conditions to yield 1-decene.
Packaging
10-Bromo-1-decene may be used to synthesize:
- 4′-(9-decenyloxy) biphenyl-4-carboxylic acid methyl ester
- 4′-(9-decenyloxy)biphenyl-4-carboxylic acid
- 9-decenylmagnesium bromide, which was employed for the preparation of (R)-tridec-12-en-2-ol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.0 °F - closed cup
Flash Point(C)
96.67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Radical initiation using borole derivatives.
Montgomery I, et al.
Tetrahedron Letters, 49(4), 628-630 (2008)
Room temperature ferroelectric terpolymers with large spontaneous polarization.
Naciri J, et al.
Macromolecules, 28(15), 5274-5279 (1995)
The four-membered-ring chemical shift anomaly.
Lambert JB, et al.
The Journal of Organic Chemistry, 48(22), 3982-3985 (1983)
Julian Gebauer et al.
The Journal of organic chemistry, 71(5), 2021-2025 (2006-02-25)
A simple access to gamma,delta-unsaturated-beta-keto lactones is presented, allowing a rapid total synthesis of the naturally occurring 16-membered macrolide antibiotic (-)-A26771B via cross metathesis, asymmetric dihydroxylation, and lactonization as the key steps.
Novel ferroelectric and electroclinic organosiloxane liquid crystals.
Naciri J, et al.
Chemistry of Materials, 7(7), 1397-1402 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service