550426
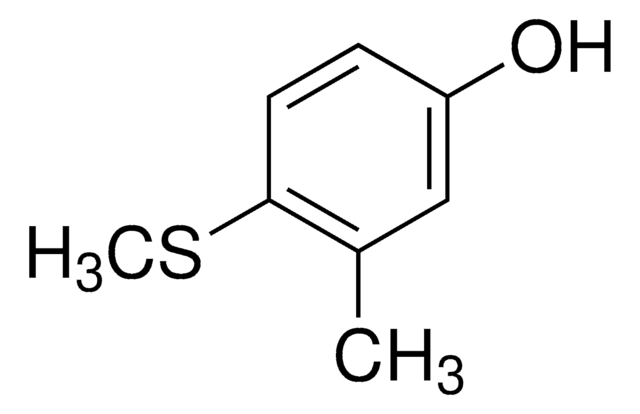
4-(Methylmercapto)phenol
98%
Synonym(s):
4-Hydroxythioanisole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3SC6H4OH
CAS Number:
Molecular Weight:
140.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
153-156 °C/20 mmHg (lit.)
mp
84-86 (lit.)
functional group
thioether
SMILES string
CSc1ccc(O)cc1
InChI
1S/C7H8OS/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3
InChI key
QASBCTGZKABPKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(Methylmercapto)phenol (4MP) mediates the removal of benzyloxycarbonyl and O-benzyl protecting groups by accepting the benzyl groups during the acidolytic cleavage with trifluoroacetic acid. The presence of hydroxyl group in the para position enhances the rate of hydrodesulfurization (HDS) of 4MP.
Application
4-(Methylmercapto)phenol [4-(Methylthio)phenol] may be used in the preparation of phosphoramidodithioate intermediates for the synthesis of sulprofos amidate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lianming Wu et al.
Journal of mass spectrometry : JMS, 44(9), 1389-1394 (2009-08-22)
A novel ion/molecule reaction was observed to occur under electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photo ionization (APPI) conditions, leading to dimerization of ionized 4-(methyl mercapto)-phenol followed by fast H(*) loss. The reaction is particularly
Acceptors in the removal of protecting groups.
Bodanszky M and Bodanszky A.
International Journal of Peptide and Protein Research, 23(3), 287-291 (1984)
Hydrotreating of compounds containing both oxygen and sulfur: effect of para-hydroxyl substituent on the reactions of mercapto and methylmercapto groups.
Viljava TR and Krause AOI.
Applied Catalysis A: General, 145(1), 237-251 (1996)
Resolution and stereoselective action of sulprofos and related S-propyl phosphorothiolates.
Hirashima A, et al.
Journal of Agricultural and Food Chemistry, 32(6), 1302-1307 (1984)
Hua Zhang et al.
Molecules (Basel, Switzerland), 15(1), 83-92 (2010-01-30)
A highly efficient transition-metal-free catalytic system Br2/NaNO2/H2O has been developed for a robust and economic acid-free aerobic oxidation of sulfides. It is noteworthy that the sulfide function reacts under mild conditions without over-oxidation to sulfone. The role of NaNO2as an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service