527750
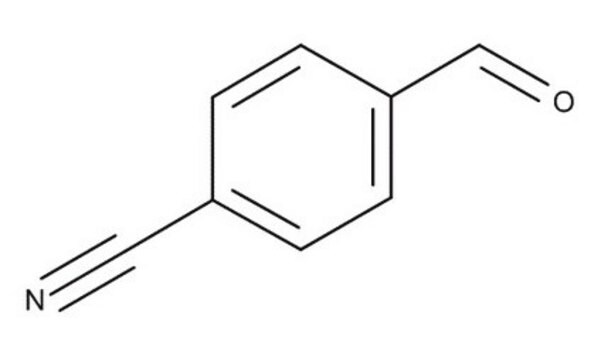
4-(Hydroxymethyl)benzonitrile
95%
Synonym(s):
4-Cyanobenzyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2C6H4CN
CAS Number:
Molecular Weight:
133.15
Beilstein:
2206193
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
39-43 °C (lit.)
functional group
hydroxyl
nitrile
SMILES string
OCc1ccc(cc1)C#N
InChI
1S/C8H7NO/c9-5-7-1-3-8(6-10)4-2-7/h1-4,10H,6H2
InChI key
XAASLEJRGFPHEV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-(Hydroxymethyl)benzonitrile can be synthesized via hydrosilylation reaction in the presence of Fe complex Bu4N[Fe(CO)3(NO)] [catalyst]. It can also be prepared from 4-(aminomethyl) benzyl alcohol.
Application
4-(Hydroxymethyl)benzonitrile [p-(hydroxymethyl)benzonitrile] may be used to synthesize p-((vinyloxy)methyl)benzonitrile (VOMBN).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bu4N [Fe (CO)3(NO)]-Catalyzed Hydrosilylation of Aldehydes and Ketones.
Dieskau AP, et al.
European Journal of Organic Chemistry, 27, 5291-5296 (2011)
Bergstra?er U, et al.
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 219-219 (2014)
Vinyl monomers bearing chromophore moieties and their polymers. VIII. synthesis and fluorescence behavior of a vinyloxy monomer having an electron-accepting chromophore moiety, p-((vinyloxy) methyl) benzonitrile, and its polymers.
Du FS, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 37(2), 179-187 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)