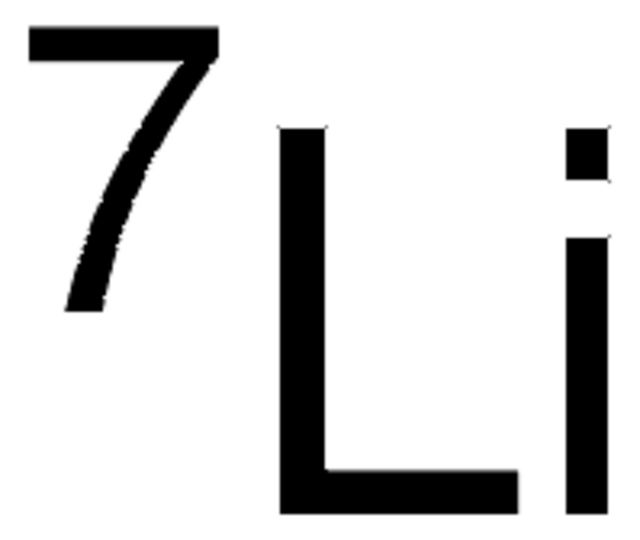499811
Lithium
granular, 99% trace metals basis
Synonym(s):
Lithium atom, Lithium element
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
Li
CAS Number:
Molecular Weight:
6.94
EC Number:
MDL number:
UNSPSC Code:
12141803
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99% trace metals basis
form
granular
reaction suitability
reagent type: reductant
resistivity
9.446 μΩ-cm, 20°C
bp
1342 °C (lit.)
mp
180 °C (lit.)
density
0.534 g/mL at 25 °C (lit.)
SMILES string
[Li]
InChI
1S/Li
InChI key
WHXSMMKQMYFTQS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Granular lithium is a form of lithium metal that has been ground into small granules. Lithium is a soft, silver-white metal that is highly reactive and flammable. Lithium has a low density of 0.534 g/cm3, which makes it one of the lightest metals. Like the other alkali metals, lithium has a low melting point (180 °C) and is a good conductor of electricity and heat. Granular lithium is highly reactive and behaves as a strong reducing agent. It must be handled with caution due to its potential to ignite or explode. Lithium reacts readily with water and oxygen to form lithium hydroxide and lithium oxide, respectively, and even reacts with nitrogen gas at room temperature, forming lithium nitride. Typically, lithium is stored in an argon glovebox and airtight containers to prevent exposure to moisture, air, and nitrogen. To make granular lithium, the metal is first extracted from ores such as spodumene or lepidolite using a process called lithium carbonate conversion. After the lithium has been purified to a high degree of purity, it is milled into granules by rotating blades.
Lithium is a low-density alkali metal that is widely used as an anode material in rechargeable and non-rechargeable batteries. It is also used to alloy with aluminum and magnesium to make them stronger and lighter.
Application
Lithium can be used as:
- A precursor to synthesize Li-based alloys such as LiAl and LiSi alloys, which are applicable as anode materials in the field of energy conversion and storage.
- A reducing agent in the reduction of zirconium oxide compounds in molten LiCl salt.
- As starting material to synthesize a reducing agent(1,4-bis(trimethylgermyl)-1.4-dihydropyrazine), for the fabrication of nickel metal films.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A low-temperature thermal ALD process for nickel utilizing dichlorobis(triethylphosphine)nickel(ii) and 1{,}4-bis(trimethylgermyl)-1{,}4-dihydropyrazine"
Anton Vihervaara,
Dalton Transactions, 51, 10898-10908 (2022)
U Eismann et al.
Optics express, 21(7), 9091-9102 (2013-04-11)
We present an all-solid-state laser source emitting up to 2.1 W of single-frequency light at 671 nm developed for laser cooling of lithium atoms. It is based on a diode-pumped, neodymium-doped orthovanadate (Nd:YVO(4)) ring laser operating at 1342 nm. Optimization
M Sathiya et al.
Nature materials, 12(9), 827-835 (2013-07-16)
Li-ion batteries have contributed to the commercial success of portable electronics and may soon dominate the electric transportation market provided that major scientific advances including new materials and concepts are developed. Classical positive electrodes for Li-ion technology operate mainly through
Enikö Zörgö et al.
PLoS genetics, 9(3), e1003388-e1003388 (2013-04-05)
The number of chromosome sets contained within the nucleus of eukaryotic organisms is a fundamental yet evolutionarily poorly characterized genetic variable of life. Here, we mapped the impact of ploidy on the mitotic fitness of baker's yeast and its never
Jian Jiang et al.
Nanoscale, 5(17), 8105-8113 (2013-07-26)
Controlled integration of multiple semiconducting oxides into each single unit of ordered nanotube arrays is highly desired in scientific research for the realization of more attractive applications. We herein report a diffusion-controlled solid-solid route to evolve simplex Co(CO3)0.5(OH)0.11H2O@TiO2 core-shell nanowire
Global Trade Item Number
| SKU | GTIN |
|---|---|
| L492728-1EA | |
| 499811-25G | 4061832422176 |
| 499811-100G | 4061832422169 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service