481122
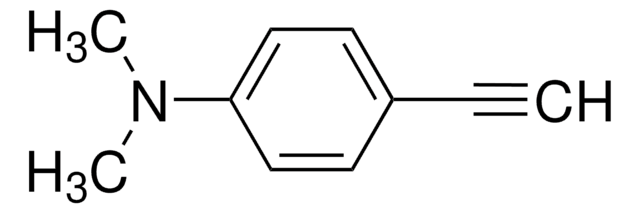
4-Ethynylaniline
97%
Synonym(s):
1-Amino-4-ethynylbenzene, P-APAC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HC≡CC6H4NH2
CAS Number:
Molecular Weight:
117.15
Beilstein:
2205181
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: click chemistry
mp
98-102 °C (dec.) (lit.)
SMILES string
Nc1ccc(cc1)C#C
InChI
1S/C8H7N/c1-2-7-3-5-8(9)6-4-7/h1,3-6H,9H2
InChI key
JXYITCJMBRETQX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Ethynylaniline, also known as p-ethynylaniline, is a terminal alkyne. Its synthesis using 2-methyl-3-butyn-2-ol (MEBYNOL) has been reported. The transition metal catalyzed polymerization of 4-ethynylaniline to afford poly(4-ethynylaniline) has been reported. The impact of the surface functionalization with 4-ethynylaniline on the thermal behavior of multi-walled carbon nanotubes (MWNTs) and graphene has been investigated.
Application
4-Ethynylaniline may be used in the synthesis of N-methyliminodiethyl 4-(4-ethynylphenyliminomethyl)benzeneboronate. It can also be used to prepare an acetylene ligand, HC2-NDI (NDI= 1,4,5,8-naphthalenediimide).
Used as an alkyne component in a synthesis of indoles from nitroarenes in the presence of a palladium-phenantroline catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fabio Ragaini et al.
The Journal of organic chemistry, 71(10), 3748-3753 (2006-05-06)
Palladium-phenanthroline complexes efficiently catalyze the reaction of nitroarenes with arylalkynes and CO to give 3-arylindoles by an ortho-C-H functionalization of the nitroarene ring. Both electron-withdrawing and electron-donating substituents are tolerated on the nitroarene, except for bromide and activated chloride. Nitroarenes
Platinum (II) phosphine complexes with acetylene ligands containing 1,4,5,8-naphthalenediimide: Synthesis, crystal structure and electrochemistry.
Shavaleev NM, et al.
Journal of Organometallic Chemistry, 692(4), 921-925 (2007)
Comparative study of the covalent diazotization of graphene and carbon nanotubes using thermogravimetric and spectroscopic techniques.
Castelain M, et al.
Physical Chemistry Chemical Physics, 15(39), 16806-16811 (2013)
Arijit Sengupta et al.
International journal of biological macromolecules, 82, 256-263 (2015-10-18)
Amphiphilic hybrid graft copolymers were synthesized using a graft-to methodology and their protein adsorption profiles studied. Three different hydrophilic side chains were studied: hydroxypropylated high amylose starch, maltodextrin, and polyethylene glycol (PEG). In the high amylose starch compositions, there was
A simple and economical synthetic route to p-ethynylaniline and ethynyl-terminated substrates.
Melissaris AP and Litt MH.
The Journal of Organic Chemistry, 59(19), 5818-5821 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service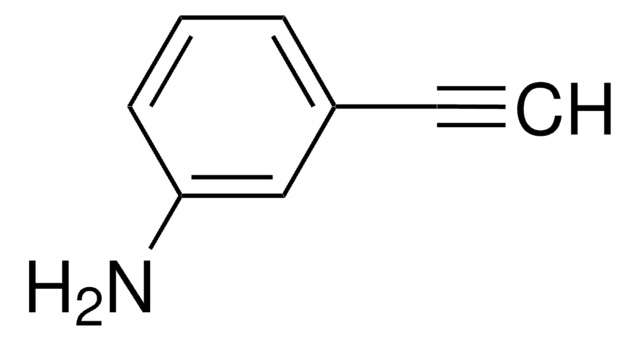


![4-[(4-Fluorophenyl)ethynyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/188/684/0b16c024-0d26-4b43-a607-60b40446e593/640/0b16c024-0d26-4b43-a607-60b40446e593.png)