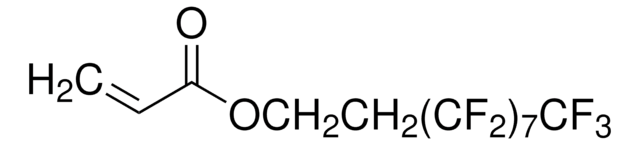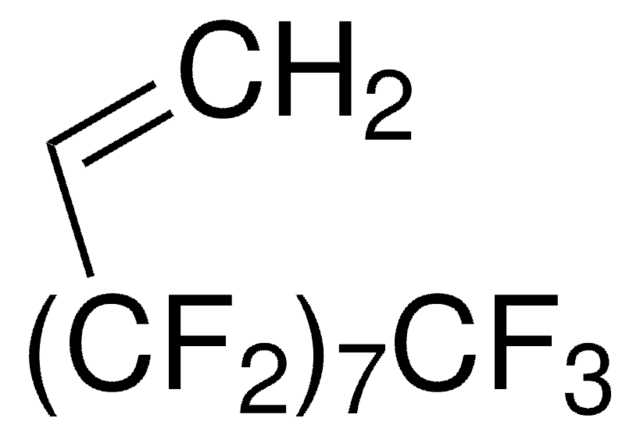474223
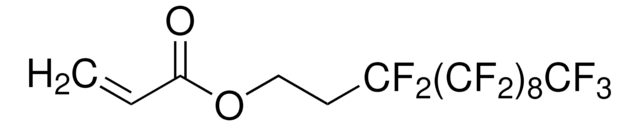
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl methacrylate
contains MEHQ as inhibitor, 97%
Synonym(s):
HDFDMA
About This Item
Recommended Products
Assay
97%
contains
MEHQ as inhibitor
refractive index
n20/D 1.343 (lit.)
bp
110 °C/4 mmHg (lit.)
density
1.596 g/mL at 25 °C (lit.)
application(s)
PFAS testing
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C14H9F17O2/c1-5(2)6(32)33-4-3-7(15,16)8(17,18)9(19,20)10(21,22)11(23,24)12(25,26)13(27,28)14(29,30)31/h1,3-4H2,2H3
InChI key
HBZFBSFGXQBQTB-UHFFFAOYSA-N
General description
Application
- As a monomer to synthesize poly(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl methacrylate) (PFDMA). PFDMA can be used to prepare a super amphiphobic sponge that repels both water and oil via the initiated chemical vapor deposition(iCVD).
- As a surface modification agent to alter the surface energy of steel-use-stainless (SUS) membrane to separate microalgal lipids from wet biomass. This helps to simplify the process of microalgae-derived biodiesel production.
- To fabricate biocompatible polymer coatings for transmucosal delivery of oral drugs. The polymer coating induces hydrophobicity that helps to avoid the release of drugs in unwanted directions.
- As a precursor/monomer to prepare triblock copolymers as electrolytes for Li metal batteries. It enhances mechanical strength, lithium-ion transference number, and cycling stability.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Lact. - Repr. 1B - STOT RE 1
Target Organs
Liver
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service