All Photos(1)
About This Item
Linear Formula:
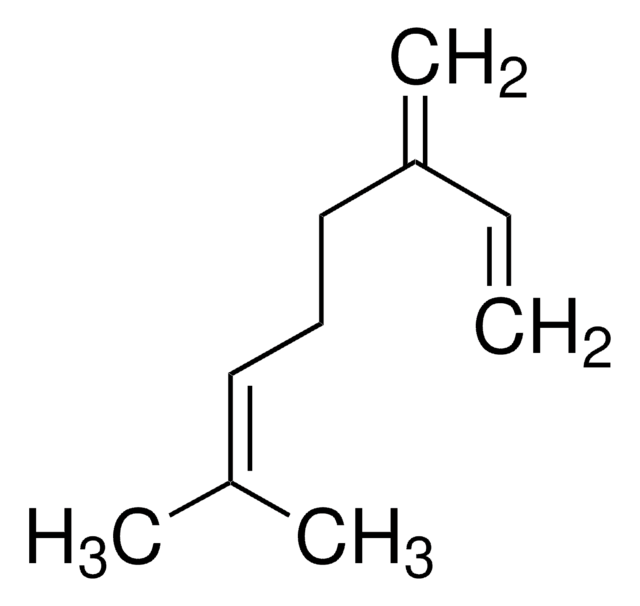
(CH3)2CHCH2CH2CH2CH(CH3)CH=CH2
CAS Number:
Molecular Weight:
140.27
Beilstein:
1719870
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
refractive index
n20/D 1.417
bp
154-156 °C (lit.)
density
0.733 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)CCCC(C)C=C
InChI
1S/C10H20/c1-5-10(4)8-6-7-9(2)3/h5,9-10H,1,6-8H2,2-4H3
InChI key
KSXTZYRIJKDCEA-UHFFFAOYSA-N
Related Categories
General description
(R)-3,7-dimethyl-1-octene is an optically active alkyl-α-olefin. Its copolymerization with styrene, 1-vinylnaphthalene or 2-methylstyrene by Ziegler-Natta catalysts has been reported. Preparation of copolymers of racemic and optically active 3,7-dimethyl-1-octene with ethylene in the presence of Ziegler-Natta isospecific catalysts have been studied. Polymerization of (R,S)-3,7-dimethyl-1-octene in the presence of heterogeneous or homogeneous isotactic specific catalysts involves highly stereospecific insertion of (R,S)-3,7-dimethyl-1-octene into the metal-CH3 bond.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Optically active hydrocarbon polymers with aromatic side chains, 7. Synthesis and properties of copolymers of vinyl aromatic monomers with chiral α-olefins.
Carlini C and Chiellini E.
Marine Chemistry, 176(3), 519-535 (1975)
Stereoselective copolymerization of racemic a-olefins with ethylene by isospecific Ziegler-Natta catalyst.
Ciardelli F, et al.
Marine Chemistry, 175(3), 923-933 (1974)
13C-enriched end groups of poly (3, 7-dimethyl-1-octene) prepared in the presence of isotactic specific catalysts.
Longo P, et al.
Macromolecular Rapid Communications, 18(6), 491-495 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service