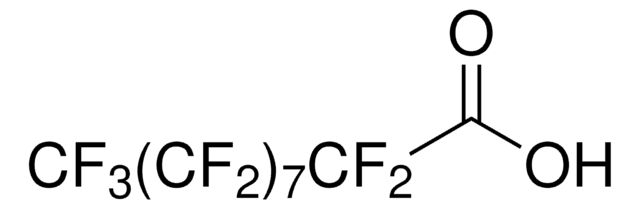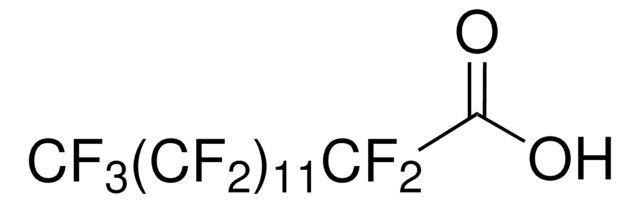406449
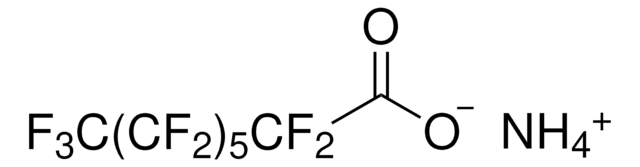
Tricosafluorododecanoic acid
95%
Synonym(s):
Perfluorododecanoic acid, Perfluorolauric acid
About This Item
Recommended Products
Assay
95%
form
solid
bp
245 °C/740 mmHg (lit.)
mp
105-108 °C (lit.)
SMILES string
OC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C12HF23O2/c13-2(14,1(36)37)3(15,16)4(17,18)5(19,20)6(21,22)7(23,24)8(25,26)9(27,28)10(29,30)11(31,32)12(33,34)35/h(H,36,37)
InChI key
CXGONMQFMIYUJR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Lact. - Repr. 1B - STOT RE 1
Target Organs
Liver
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service