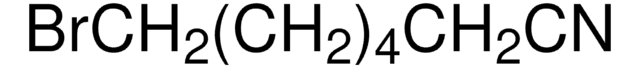392278
6-Bromohexanenitrile
95%
Synonym(s):
5-Bromopentyl cyanide, 6-Bromocapronitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)5CN
CAS Number:
Molecular Weight:
176.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.477 (lit.)
bp
134 °C/13 mmHg (lit.)
density
1.328 g/mL at 25 °C (lit.)
functional group
bromo
nitrile
SMILES string
BrCCCCCC#N
InChI
1S/C6H10BrN/c7-5-3-1-2-4-6-8/h1-5H2
InChI key
PHOSWLARCIBBJZ-UHFFFAOYSA-N
General description
6-Bromohexanenitrile (6-Bromohexanonitrile) is an ω-bromoalkanonitrile. Friedel Crafts alkylation of 6-bromohexanenitrile with benzene has been studied.
Application
6-Bromohexanenitrile (6-Bromocapronitrile, 6-Bromohexanonitrile) is suitable reagent used in the synthesis of (5-cyanopentyl)zinc(II) bromide, an organozinc reagent. It may be used in the synthesis of the following:
- 6-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)hexanenitrile.
- dimethyl 6,6′-dithiobiscaproimidate, a long-chain dithiobisimidate.
- N-benzyloxy-(4-cyanopentyl)-carbamic acid ethyl ester, a N-benzyloxy carbamate derivative.
- 1-hetarylsulfanyl-ω-cyanoalkanes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Friedel-Crafts alkylation of benzene by normal ?-chloroalkanoic acids and their methyl esters and nitriles.
Zakharkin LI and Anikina EV
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 36(2), 327-330 (1987)
H Peretz et al.
European journal of biochemistry, 63(1), 77-82 (1976-03-16)
This communication describes a simple method for synthesizing cleavable bifunctional imido esters of different chain lengths. These reagents, which form covalent crosslinks between lysine residues of proteins, contain a disulfide bond which is cleaved under mild conditions by reducing agents
?Green Chemical" Methods for the Regioselective Synthesis of 1-Hetarylsulfanyl-?-Cyanoalkanes."
Abele E, et al.
Latvijas Kimijas Zurnals, 49(1), 278-282 (2010)
NICKEL-CATALYZED ENANTIOSELECTIVE NEGISHI CROSS-COUPLINGS OF RACEMIC SECONDARY α-BROMO AMIDES WITH ALKYLZINC REAGENTS: (S)-N-BENZYL-7-CYANO-2-ETHYL-N-PHENYLHEPTANAMIDE.
Sha Lou et al.
Organic syntheses; an annual publication of satisfactory methods for the preparation of organic chemicals, 87, 330-330 (2010-01-01)
Yuan Liu et al.
Tetrahedron, 67(12), 2206-2214 (2011-04-19)
N-Alkyl-N-benzyloxy carbamates, 2, undergo facile intramolecular cyclization with a variety of carbon nucleophiles to give functionalized 5- and 6-membered protected cyclic hydroxamic acids, 3, in good to excellent yields. This method can be extended to prepare seven-membered cyclic hydroxamic acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service