280992
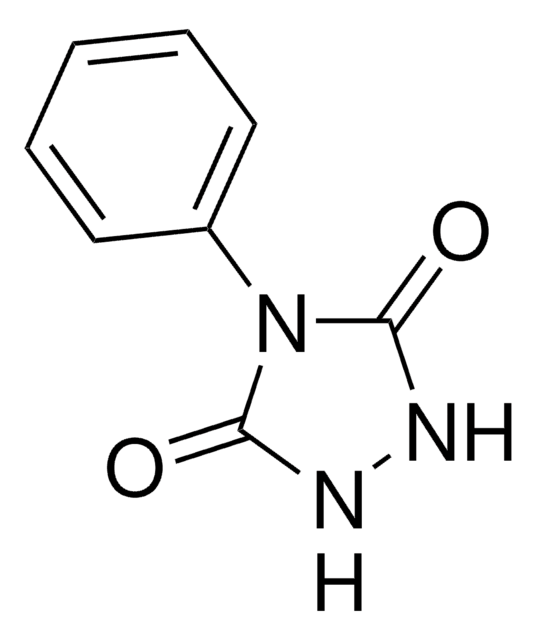
4-Phenyl-1,2,4-triazoline-3,5-dione
97%
Synonym(s):
4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione, PTAD
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H5N3O2
CAS Number:
Molecular Weight:
175.14
Beilstein:
141548
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
165-170 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
O=C1N=NC(=O)N1c2ccccc2
InChI
1S/C8H5N3O2/c12-7-9-10-8(13)11(7)6-4-2-1-3-5-6/h1-5H
InChI key
ISULLEUFOQSBGY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) can be used as an efficient and selective reagent for the oxidation of thiols to disulfides.
It can also be used:
It can also be used:
- As a dehydrogenating agent to synthesize annulated dihydropyridazines by inverse [4+2] cycloaddition reaction between cyclic alkenes and 1,2,4,5-tetrazines.
- As a dienophile to synthesize cycloaddition products by fast hetero-Diels−Alder reactions.
- As an efficient oxidizing agent for the synthesis of pyridine derivatives from 1,4-dihydropyridines.
- In the synthesis of urazoles via [3+2] cycloaddition with allylsilanes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Location of conjugated diene position in an aliphatic chain by mass spectrometry of the 4-phenyl-1,2,4-triazoline-3,5-dione adduct.
D C Young et al.
Analytical chemistry, 59(15), 1954-1957 (1987-08-01)
Tatsuya Higashi et al.
Analytical and bioanalytical chemistry, 403(2), 495-502 (2012-03-01)
The utility of Diels-Alder derivatization with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) for liquid chromatography/electrospray ionization tandem mass spectrometry of conjugated linoleic acids (CLAs) was examined. PTAD rapidly reacted with the CLAs, and the resulting derivatives were highly responsive in electrospray ionization mass spectrometry
Tetrahedron Letters, 48, 6671-6671 (2007)
4-Phenyl-1, 2, 4-triazole-3, 5-dione as a novel and reusable reagent for the aromatization of 1, 4-dihydropyridines under mild conditions
Zolfigol MA, et al.
Tetrahedron Letters, 46(33), 5581-5584 (2005)
K Søndergaard et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 7(11), 2324-2331 (2001-07-12)
The 5-aza-6-deoxy analogue of castanospermine (+/-)-5a and its 1-epimer (+/-)-5b was synthesized. The synthesis started from the known compound 5-benzyloxy-7-hydroxyhepta-1,3-diene, which was protected and subjected to Diels-Alder reaction with 4-phenyl-1,2,4-triazoline-3,5-dione to give two epimeric adducts. One of these was transformed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)

