193526
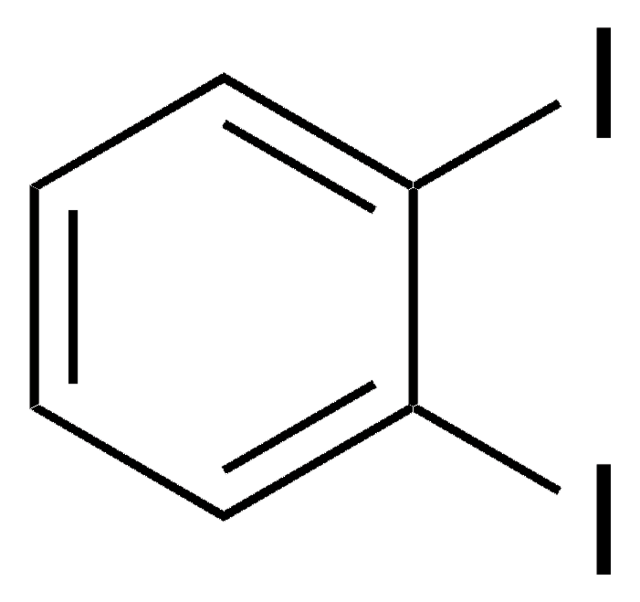
1,4-Diiodobenzene
99%
Synonym(s):
4-Iodophenyl iodide, p-Diiodobenzene, p-Phenylene diiodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4I2
CAS Number:
Molecular Weight:
329.90
Beilstein:
1904546
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
bp
285 °C (lit.)
mp
131-133 °C (lit.)
SMILES string
Ic1ccc(I)cc1
InChI
1S/C6H4I2/c7-5-1-2-6(8)4-3-5/h1-4H
InChI key
LFMWZTSOMGDDJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,4-Diiodobenzene was used in:
- total synthesis of martinellic acid, a naturally occurring bradykinin receptor antagonist
- preparation of 1,4-bis(p-R-phenylethynyl)benzenes via Pd11/Cu1catalyzed cross-coupling reaction
- synthesis of 1,4-diiodo-2,5-didodecylbenzene, starting reagent for the preparation of oligo(1,4-phenylene ethynylene)s
- surface-mediated synthesis of epitaxially aligned and separated polyphenylene lines on Cu(110) via Ullmann dehalogenation reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of symmetric and unsymmetric 1, 4-bis ( p-R-phenylethynyl) benzenes via palladium/copper catalyzed cross-coupling and comments on the coupling of aryl halides with terminal alkynes.
Nguyen P, et al.
Inorgorganica Chimica Acta, 220(1), 289-296 (1994)
D Ma et al.
Organic letters, 3(14), 2189-2191 (2001-07-07)
[reaction: see text] The first total synthesis of martinellic acid, a naturally occurring bradykinin receptor antagonist, via a CuI-catalyzed coupling reaction of beta-amino ester 6 with 1,4-diiodobenzene and a guanylation reaction of secondary amine 3 under mild conditions as key
Fabrication of a conjugated microporous polymer membrane and its application for membrane catalysis.
Jieun Lee et al.
Scientific reports, 7(1), 13568-13568 (2017-10-21)
A flexible and free standing conjugated microporous polymer (CMP) membrane was prepared using a polyvinylpyrrolidone (PVP) electrospun membrane as a template. The PVP nanofibers of the template membrane were coated with a thin layer of the CMP through the in
J A Lipton-Duffin et al.
Small (Weinheim an der Bergstrasse, Germany), 5(5), 592-597 (2009-02-26)
The surface-mediated synthesis of epitaxially aligned and separated polyphenylene lines on Cu(110) by exploiting the Ullmann dehalogenation reaction is reported. Scanning tunneling microscopy (STM) and X-ray photoelectron spectroscopy (XPS) show that the C-I bonds of 1,4-diiodobenzene and 1,3-diiodobenzene (C(6)H(4)I(2)) are
Uta Funke et al.
Pharmaceuticals (Basel, Switzerland), 5(2), 169-188 (2012-01-01)
Phosphodiesterase 10A (PDE10A) is a key enzyme of intracellular signal transduction which is involved in the regulation of neurotransmission. The molecular imaging of PDE10A by PET is expected to allow a better understanding of physiological and pathological processes related to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service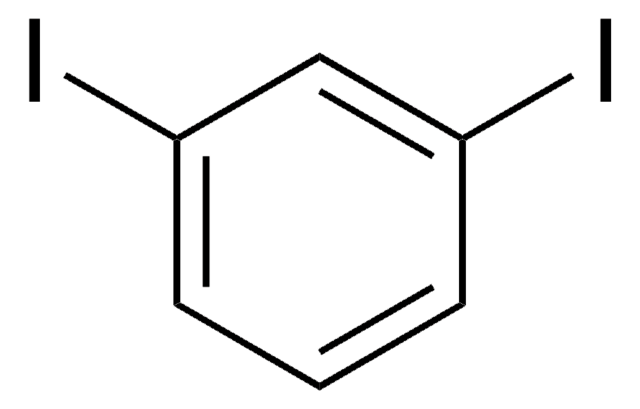



![des-Arg9-[Leu8]-Bradykinin acetate salt ≥97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/349/954/df5fe325-b9df-4769-80d8-10a3c754de46/640/df5fe325-b9df-4769-80d8-10a3c754de46.png)