126209
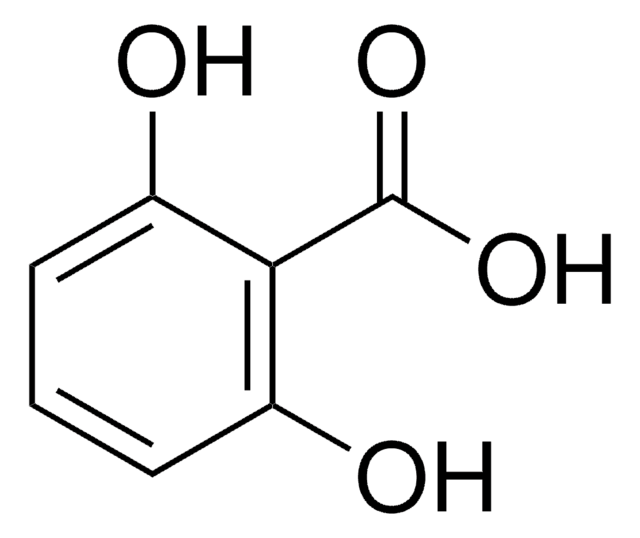
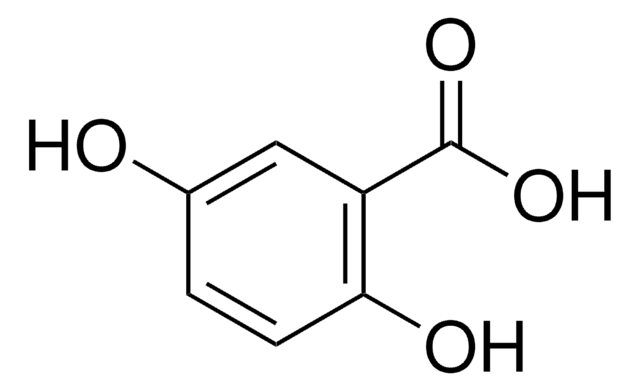
2,3-Dihydroxybenzoic acid
99%
Synonym(s):
Hypogallic acid, Pyrocatechuic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
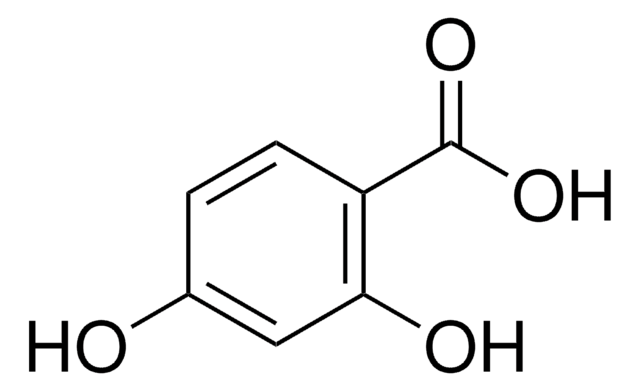
(HO)2C6H3CO2H
CAS Number:
Molecular Weight:
154.12
Beilstein:
2209117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
204-206 °C (lit.)
solubility
methanol: soluble 50 mg/mL, clear to faintly hazy, orange
functional group
carboxylic acid
SMILES string
OC(=O)c1cccc(O)c1O
InChI
1S/C7H6O4/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3,8-9H,(H,10,11)
InChI key
GLDQAMYCGOIJDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,3-Dihydroxybenzoic acid was used to study the complexes of manganese with aliphatic and aromatic polyhydroxy ligands in basic media by electrochemical, spectrophotometric and magnetic methods. It was used in isolation of new siderophore named vulnibactin from low iron cultures of Vibrio vulnificus, a human pathogen. It was used as cocrystal former to study the influence of position isomerism on the co-crystals formation and physicochemical properties of piracetam.
Biochem/physiol Actions
2,3-Dihydroxybenzoic acid was found to be orally effective iron-chelating drug in hypertransfused rat model. It is monocatechol siderophore produced by Brucella abortus.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiao Jia et al.
Journal of medicinal chemistry, 63(11), 6003-6027 (2020-05-19)
We disclose a study on nucleoside triphosphate (NTP) analogues in which the γ-phosphate is covalently modified by two different biodegradable masking units and d4T as nucleoside analogue that enable the delivery of d4TTP with high selectivity in phosphate buffer (pH
Tomokazu Takano et al.
PloS one, 11(10), e0165424-e0165424 (2016-10-28)
Erythrocytic inclusion body syndrome (EIBS) causes mass mortality in farmed salmonid fish, including the coho salmon, Onchorhynchus kisutchi, and chinook salmon, O. tshawytscha. The causative agent of the disease is a virus with an icosahedral virion structure, but this virus
Barry Goodell et al.
Biotechnology for biofuels, 10, 179-179 (2017-07-14)
Wood decayed by brown rot fungi and wood treated with the chelator-mediated Fenton (CMF) reaction, either alone or together with a cellulose enzyme cocktail, was analyzed by small angle neutron scattering (SANS), sum frequency generation (SFG) spectroscopy, Fourier transform infrared
Polarographic and spectroscopic studies of the manganese (II),-(III), and-(IV) complexes formed by polyhydroxy ligands.
Magers KD, et al.
Inorganic Chemistry, 17(3), 515-523 (1978)
Bryan H Bellaire et al.
Infection and immunity, 71(4), 1794-1803 (2003-03-26)
Brucella abortus reportedly produces the monocatechol siderophore 2,3-dihydroxybenzoic acid (2,3-DHBA) in response to iron limitation. Nucleotide sequence analysis of the cloned DHBA biosynthesis locus from virulent B. abortus 2308 and genetic complementation of defined Escherichia coli mutants were used to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)