108146
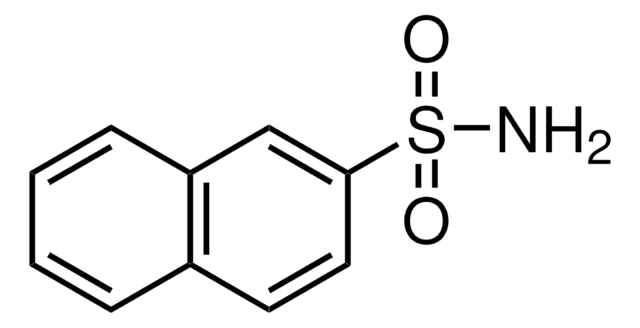
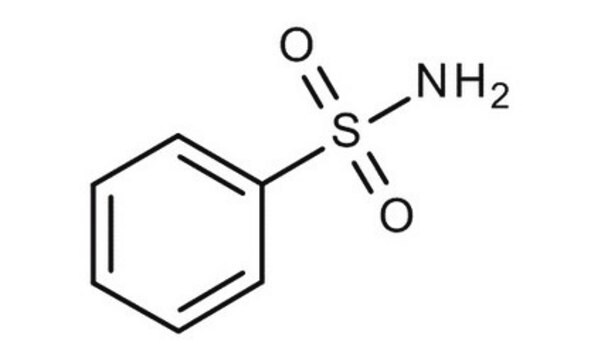
Benzenesulfonamide
≥98%
Synonym(s):
Phenylsulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SO2NH2
CAS Number:
Molecular Weight:
157.19
Beilstein:
1100566
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39093209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
solid
mp
149-152 °C (lit.)
solubility
methanol: soluble 25 mg/mL
SMILES string
NS(=O)(=O)c1ccccc1
InChI
1S/C6H7NO2S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H,(H2,7,8,9)
InChI key
KHBQMWCZKVMBLN-UHFFFAOYSA-N
Gene Information
human ... CA1(759)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Benzenesulfonamide was used to develop analytical method for simultaneous determination of benzotriazole, benzothiazole and benzenesulfonamide contaminants in environmental waters.
Biochem/physiol Actions
Benzenesulfonamide is an inhibitor of human carbonic anhydrase B. Benzenesulfonamide derivatives are effective in the treatment of proliferative diseases such as cancer. It is used in the synthesis of dyes, photochemicals and disinfectants.
Preparation Note
Benzenesulfonamide dissolves in methanol at a concentration of 25 mg/ml to form a clear, colourless solution.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rafia Bashir et al.
Bioorganic & medicinal chemistry letters, 21(14), 4301-4305 (2011-06-15)
Thirteen new 2-pyrazoline derivatives bearing benzenesulfonamide moiety (2a-m) were synthesized by condensing appropriate chalcones with 4-hydrazinonbenzenesulfonamide hydrochloride and tested for anticancer and anti-inflammatory actions. According to the protocol of the National Cancer Institute (NCI) in vitro disease-oriented human cells screening
Astrid Goubet et al.
Bioorganic & medicinal chemistry letters, 23(3), 761-763 (2012-12-26)
C5-Ethynylbenzenesulfonamide-modified nucleotide (EBNA) was investigated as substrate of various DNA polymerases. The experiments revealed that KOD, Phusion and Klenow DNA polymerases successfully accepted EBNA-T nucleotide as a substrate and yielded the fully extended DNA. KOD DNA polymerase was found to
Design of [(2-pyrimidinylthio)acetyl]benzenesulfonamides as inhibitors of human carbonic anhydrases.
Edita Čapkauskaitė et al.
European journal of medicinal chemistry, 51, 259-270 (2012-03-24)
A series of [(2-pyrimidinylthio)acetyl]benzenesulfonamides were designed and synthesized. Their binding affinities as inhibitors of several recombinant human carbonic anhydrase (CA) isozymes were determined by isothermal titration calorimetry (ITC) and thermal shift assay (TSA). A group of compounds containing a chlorine
Asha Chandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 92, 84-90 (2012-03-27)
The FT-IR and FT-Raman spectra of (E)-N-carbamimidoyl-4-((4-methoxybenzylidene)amino)benzenesulfonamide were recorded and analyzed. Geometry and harmonic vibrational wavenumbers were calculated theoretically using Gaussian 03 set of quantum chemistry codes. Calculations were performed at the Hartree-Fock (HF) and density functional theory (DFT) levels
Nitrogen-15 nuclear magnetic resonance study of benzenesulfonamide and cyanate binding to carbonic anhydrase.
K Kanamori et al.
Biochemistry, 22(11), 2658-2664 (1983-05-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)