All Photos(1)
About This Item
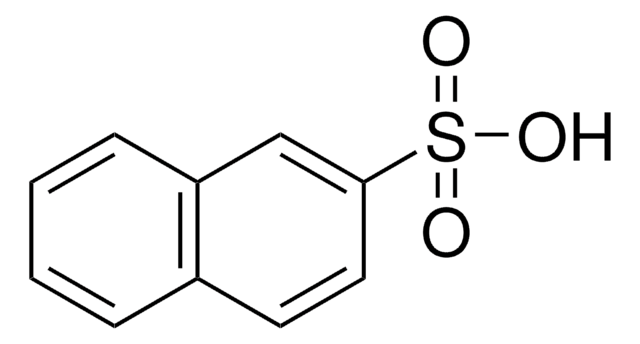
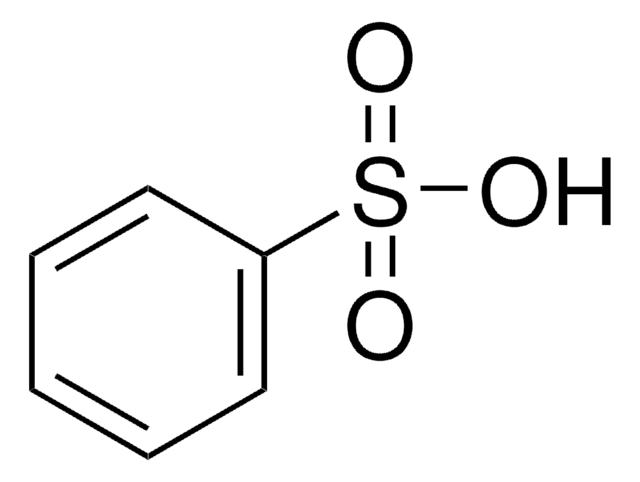
Linear Formula:
C10H7SO3H
CAS Number:
Molecular Weight:
208.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
concentration
>50%
mp
77-79 °C (lit.)
solubility
alcohol: freely soluble
diethyl ether: slightly soluble
water: freely soluble
SMILES string
OS(=O)(=O)c1cccc2ccccc12
InChI
1S/C10H8O3S/c11-14(12,13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,11,12,13)
InChI key
PSZYNBSKGUBXEH-UHFFFAOYSA-N
Gene Information
human ... EGFR(1956) , LCK(3932)
General description
Mechanism of metabolism of 1-naphthalenesulfonic acid by green algae Scenedesmus obliquus has been investigated.
Application
1-Naphthalenesulfonic acid was used as template molecule to prepare new non-covalent molecularly imprinted polymer for solid-phase extraction of naphthalene sulfonates.
Other Notes
remainder naphthalenesulfonic acid, sulfuric acid and water
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reddicherla Umapathi et al.
Colloids and surfaces. B, Biointerfaces, 135, 588-595 (2015-09-01)
A lack of sufficient knowledge regarding the behaviour of stimuli-responsive polymers to biological stimuli hinders the potential use of responsive polymers as biomaterials and medical devices. Hence, in this study, we demonstrate the impact of various globular proteins on the
Jiao Guan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 202, 1-12 (2018-05-20)
The antimicrobial triclocarban (TCC) is frequently found in various personal care products (PCPs), and recent studies have demonstrated that it shows a high unintended biological activity on humans and wildlife. To evaluate the toxicity of TCC at the protein level
Yan Gong et al.
International journal of biological macromolecules, 101, 32-39 (2017-03-23)
The α-glucosidase inhibitor is of interest to researchers due to its association with type-2 diabetes treatment. Hesperetin is a flavonoid with natural antioxidant properties. This paper presents an evaluation on the effects of hesperetin on α-glucosidase via inhibitory kinetics using
Haibin Luo et al.
Journal of chromatography. A, 1424, 92-101 (2015-11-26)
We have systemically investigated unusual elution behaviors of an IgG4 (mAb A) in cation exchange chromatography (CEX). This mAb A exhibited two elution peaks under certain conditions when being purified by several strong CEX columns. When either of the two
Ester Caro et al.
Journal of chromatography. A, 1047(2), 175-180 (2004-10-06)
A new polymeric sorbent synthesised by exploiting molecular imprinting technology has been used to selectively extract naphthalene sulfonates (NSs) directly from aqueous samples. In the non-covalent molecular imprinting approach used to prepare this polymer, 1-naphthalene sulfonic acid (1-NS) and 4-vinylpyridine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service