Alle Fotos(1)
Wichtige Dokumente
S5696
SKI II
≥98% (HPLC), solid
Synonym(e):
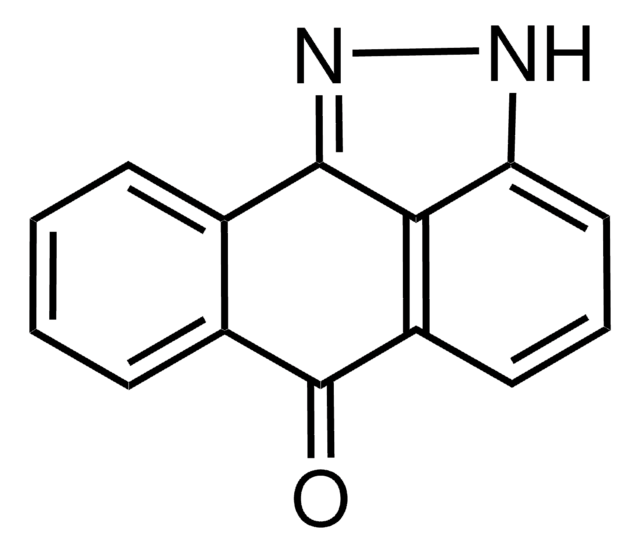
4-[[4-(4-Chlorophenyl)-2-thiazolyl]amino]phenol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C15H11ClN2OS
CAS-Nummer:
Molekulargewicht:
302.78
MDL-Nummer:
UNSPSC-Code:
12352200
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Assay
≥98% (HPLC)
Form
solid
Lagerbedingungen
protect from light
Farbe
off-white
Löslichkeit
DMSO: ≥20 mg/mL
Lagertemp.
2-8°C
SMILES String
Oc1ccc(Nc2nc(cs2)-c3ccc(Cl)cc3)cc1
InChI
1S/C15H11ClN2OS/c16-11-3-1-10(2-4-11)14-9-20-15(18-14)17-12-5-7-13(19)8-6-12/h1-9,19H,(H,17,18)
InChIKey
ZFGXZJKLOFCECI-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
Human cisplatin-resistant gastric cancer cell line was treated with SKI-II to study the mechanism of reversion of multidrug resistance.1
SKI II has been used to study the regulatory mechanism and signaling pathway induced by SKI II in inhibiting tumor growth.
Biochem./physiol. Wirkung
SKI II is a selective non-lipid sphingosine kinase (SK) inhibitor. IC50 = 0.5 μM; does not act at ATP-binding site. Displays no inhibition of ERK2, PI 3-kinase, or PKCα at concentrations up to 60 μM. Induces apoptosis and inhibits proliferation in several other tumor cell lines in vitro (IC50 = 0.9-4.6 μM).
Sphingosine kinase (SK) plays a pivotal role in regulating tumor growth and SK can act as an oncogene. Expression of SK RNA is significantly elevated in a variety of solid tumors, compared with normal tissue from the same patient. A number of novel inhibitors of human SK were identified, and several representative compounds were characterized in detail. These compounds demonstrated activity at sub- to micromolar concentrations, making them more potent than any other reported SK inhibitor, and were selective toward SK compared with a panel of human lipid and protein kinases. Kinetic studies revealed that the compounds were not competitive inhibitors of the ATP-binding site of SK. 4-[4-(4-chloro-phenyl)-thiazol-2-ylamino]-phenol (SKI-II) inhibitor is orally bioavailable, detected in the blood for at least 8 h, and showed a significant inhibition of tumor growth in mice with IC50 = 0.5 μM; SKI II does not act at ATP-binding site. Displays no inhibition of ERK2, PI 3-kinase, or PKCa at concentrations up to 60 μM.SKI II induces apoptosis and inhibits proliferation in several other tumor cell lines in vitro (IC50 = 0.9-4.6 μM).
Leistungsmerkmale und Vorteile
This compound is a featured product for Kinase Phosphatase Biology research. Click here to discover more featured Kinase Phosphatase Biology products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Sonstige Hinweise
Product is air and light sensitive
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Aleksandra M Ochnik et al.
BMC cancer, 17(1), 820-820 (2017-12-07)
Targeting the type 1 insulin-like growth factor receptor (IGF1R) in breast cancer remains an ongoing clinical challenge. Oncogenic IGF1R-signaling occurs via activation of PI3K/AKT/MAPK downstream mediators which regulate cell proliferation and protein synthesis. To further understand IGF1R signaling we have
Antimalarial drugs trigger lysosome-mediated cell death in chronic lymphocytic leukemia (CLL) cells.
Subhadip Das et al.
Leukemia research, 70, 79-86 (2018-06-15)
Lysosomes are the most acidic vesicles within mammalian cells and are promising targets for the treatment of breast cancer, glioblastomas and acute myeloid leukemia (AML). Our previous studies have shown that chronic lymphocytic leukemia (CLL) cells are also sensitive to
Zu-An Zhu et al.
Asian Pacific journal of cancer prevention : APJCP, 13(2), 625-631 (2012-04-25)
In order to investigate whether SKI-II could reverse drug resistance and its possible mechanisms, we treated SGC7901/DDP cells with SKI-II or SKI-II in combination with DDP. Then cell growth, apoptosis, micro- morphological changes, and expression of SphK1, P-gp, NF-kB, Bcl-2
Xiaobo Yang et al.
Bioelectromagnetics, 40(3), 180-187 (2019-03-29)
Previously, we found that exposure to a 50-Hz magnetic field (MF) could induce human amniotic epithelial (FL) cell proliferation and sphingosine kinase 1 (SK1) activation, but the mechanism was not clearly understood. In the present study, the possible signaling pathways
Bo Kyung A Seong et al.
Cancer research, 77(3), 696-706 (2016-12-03)
Metastatic relapse is the major cause of death in pediatric neuroblastoma, where there remains a lack of therapies to target this stage of disease. To understand the molecular mechanisms mediating neuroblastoma metastasis, we developed a mouse model using intracardiac injection
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.