P9248
PD 169316
≥98% (HPLC), solid
Synonym(e):
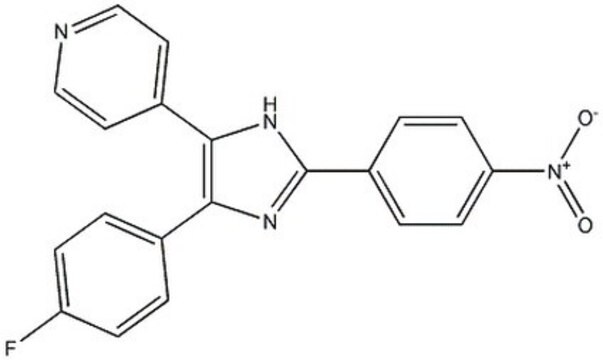
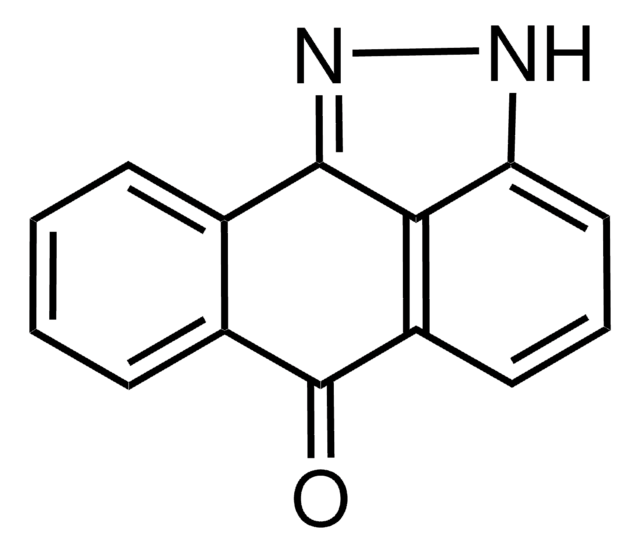
4-(4-Fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
solid
Farbe
faint yellow to dark orange
Löslichkeit
DMSO: >10 mg/mL
H2O: insoluble
Ersteller
GlaxoSmithKline
Lagertemp.
2-8°C
SMILES String
[O-][N+](=O)c1ccc(cc1)-c2nc(-c3ccc(F)cc3)c([nH]2)-c4ccncc4
InChI
1S/C20H13FN4O2/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(8-4-15)25(26)27/h1-12H,(H,23,24)
InChIKey
BGIYKDUASORTBB-UHFFFAOYSA-N
Angaben zum Gen
human ... MAPK14(1432)
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.