N9284
Nitroreductase from Escherichia coli
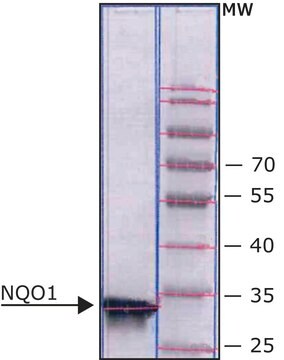
≥90% (SDS-PAGE), recombinant, expressed in E. coli
Synonym(e):
NTRA
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(4)
About This Item
Empfohlene Produkte
Rekombinant
expressed in E. coli
Qualitätsniveau
Assay
≥90% (SDS-PAGE)
Form
lyophilized powder
Spezifische Aktivität
≥100 units/mL
Mol-Gew.
monomer 24000
Grünere Alternativprodukt-Eigenschaften
Waste Prevention
Safer Solvents and Auxiliaries
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
UniProt-Hinterlegungsnummer
Grünere Alternativprodukt-Kategorie
Versandbedingung
wet ice
Lagertemp.
−20°C
Angaben zum Gen
Escherichia coli K12 ... nfsB(945483)
Allgemeine Beschreibung
Nitroreductase is a flavoprotein and is encoded by the NfsB gene. It comprises a dimer, with 217 amino acids and active site in each subunit. The structure has FMN and the substrate bound to the enzyme.
Anwendung
Nitroreductase from Escherichia coli has been used in the conjugation generation with pig liver esterase (PLE). It has also been used in chemiluminescence response studies with probes HyCL-3 and HyCL-4-AM in rat liver microsomes.
Biochem./physiol. Wirkung
Nitroreductase (NTR) catalyzes the reduction of nitroaromatic substrates and quinones. The mutant F124K of NTR is useful in cancer therapy and improves sensitization of drug CB1954.
Nitroreductase increases the sensitivity of organisms to nitro-containing drugs such as metronidazole by converting the nitro group to a cytotoxic nitro radical.
Nitroreductases can play a crucial role in redox systems via NADPH or NADH as a reductant.
Shows ability to reduce quinines. Enzyme for activating prodrugs in antibody directed enzyme prodrug therapy.
Physikalische Eigenschaften
Lyophilized powder containing PBS. Does not contain BSA as excipient
Einheitendefinition
One unit will reduce one μmole of Cytochrome C per minute in the presence of Menadione and NADH at pH 7.4 at 37 °C.
Angaben zur Herstellung
Produced using animal component-free materials.
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Dual enzyme-responsive ?turn-on? fluorescence sensing systems based on in situ formation of 7-hydroxy-2-iminocoumarin scaffolds
Debieu, S and Romieu A
Organic & Biomolecular Chemistry, 13(41), 10348-10361 (2015)
Chih-Chen Chen et al.
Food chemistry, 135(4), 2708-2713 (2012-09-18)
Nitroreductases (Nrs) play important roles in redox system via NADPH or NADH as a reductant. A TcNr cDNA encoding a putative Nr was cloned from Taiwanofungus camphorata. A 3-D structural model of the TcNr has been created based on the
Mansooreh Jaberipour et al.
Biochemical pharmacology, 79(2), 102-111 (2009-08-12)
Prodrug activation gene therapy for cancer involves expressing prodrug-activating enzymes in tumour cells, so they can be selectively killed by systemically administered prodrug. For example, Escherichia colinfsB nitroreductase (E.C. 1.6.99.7)(NTR), sensitises cells to the prodrug CB1954 (5-[aziridin-1-yl]-2,4-dinitrobenzamide), which it converts
Bharat Bhushan et al.
Biochemical and biophysical research communications, 322(1), 271-276 (2004-08-18)
Previously, we reported that a salicylate 1-monooxygenase from Pseudomonas sp. ATCC 29352 biotransformed CL-20 (2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaaza-isowurtzitane) (C(6)H(6)N(12)O(12)) and produced a key metabolite with mol. wt. 346 Da corresponding to an empirical formula of C(6)H(6)N(10)O(8) which spontaneously decomposed in aqueous medium to
M J Lemmon et al.
Gene therapy, 4(8), 791-796 (1997-08-01)
A fundamental obstacle in gene therapy for cancer treatment is the specific delivery of an anticancer gene product to a solid tumor. Although several strategies exist to control gene expression once a vector is directly introduced into a tumor, as
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![Nitrat-Reduktase (NAD[P]H) aus Aspergillus niger lyophilized powder, ≥300 units/g solid](/deepweb/assets/sigmaaldrich/product/images/309/282/2a67ae4d-ca55-4f0b-96ec-34748ff8a21e/640/2a67ae4d-ca55-4f0b-96ec-34748ff8a21e.jpg)