Alle Fotos(2)
Wichtige Dokumente
N2881
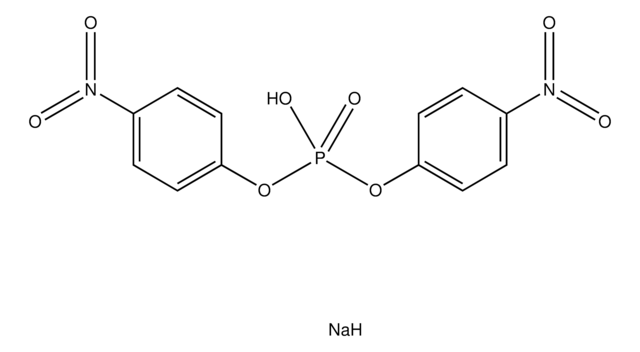
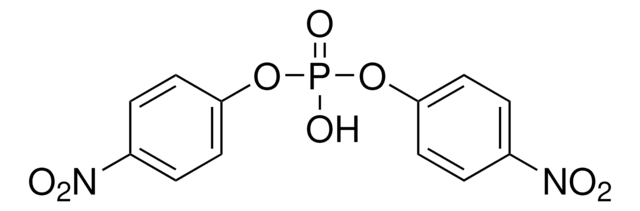
4-Nitrophenyl phenylphosphonate
adenosine receptor agonist, 5′-Nucleotide Phosphodiesterase substrate
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C12H10NO5P
CAS-Nummer:
Molekulargewicht:
279.19
MDL-Nummer:
UNSPSC-Code:
12352204
PubChem Substanz-ID:
NACRES:
NA.32
Empfohlene Produkte
Assay
≥98% (TLC)
Form
powder
Löslichkeit
acetone: 50 mg/mL, clear, faintly yellow
Lagertemp.
−20°C
SMILES String
OP(=O)(Oc1ccc(cc1)N(=O)=O)c2ccccc2
InChI
1S/C12H10NO5P/c14-13(15)10-6-8-11(9-7-10)18-19(16,17)12-4-2-1-3-5-12/h1-9H,(H,16,17)
InChIKey
NRGZTHQFAQCJCQ-UHFFFAOYSA-N
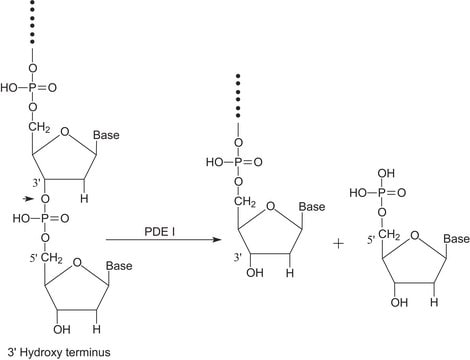
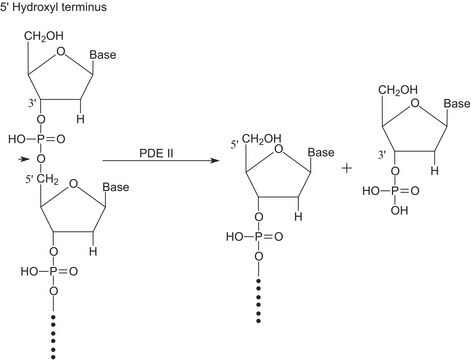
Substrate
5′-Nucleotide Phosphodiesterase substrate
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Hydrolysis of a phosphonate ester catalyzed by an enzyme from Dictyostelium discoideum.
E F Rossomando et al.
Archives of biochemistry and biophysics, 197(1), 364-366 (1979-10-01)
H J Deussen et al.
Bioorganic & medicinal chemistry, 8(3), 507-513 (2000-03-25)
A bifunctional activity label (8) for directed molecular evolution of lipolytic enzymes has been designed and synthesized. The structure is composed of a 4-nitrophenyl activated phosphonate, that is, a suicide substrate of lipases/esterases, connected to a biotin moiety through a
M Labadie et al.
Biochimie, 61(9), 1091-1094 (1979-01-01)
A "Batch" microcalorimeter is used at 30 degrees C for the study of the hydrolysis of 4-nitro-phenylphenylphosphonate with a calf-intestinal phosphonate esterase, in a tris buffer, pH 8. The yield of enzymatic hydrolysis is estimated by spectrophotometric determination of the
O A Moe et al.
The Journal of biological chemistry, 258(11), 6941-6946 (1983-06-10)
Extensive kinetic studies of bovine intestinal 5'-nucleotide phosphodiesterase as a function of pH have confirmed and amplified the catalytic mechanism previously proposed on the basis of isolation of a covalent phosphorylated intermediate (Landt, M., and Butler, L.G. (1978) Biochemistry 17
A G Wang et al.
Ophthalmology, 106(7), 1287-1291 (1999-07-16)
To determine the cerebral metabolism of patients with cortical visual loss. Two observational case studies. Two patients who survived acute organophosphate poisoning with respiratory failure experienced severe visual loss despite relatively normal ophthalmic examination results. Magnetic resonance imaging of the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.