Alle Fotos(2)
Wichtige Dokumente
H7021
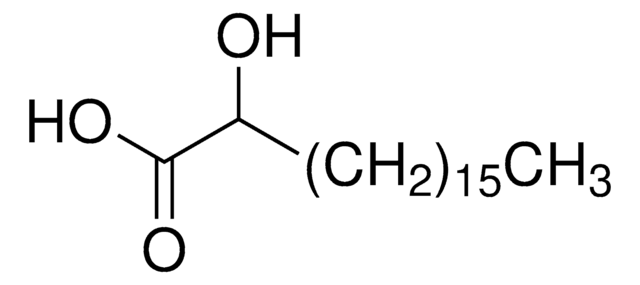
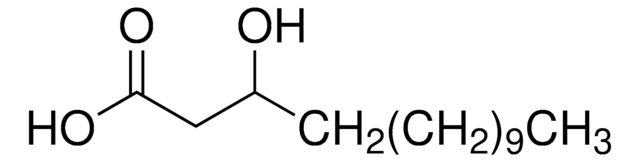
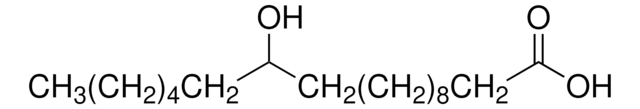
2-Hydroxyhexadecansäure
≥98% (capillary GC)
Synonym(e):
2-Hydroxy-palmitinsäure
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
CH3(CH2)13CH(OH)COOH
CAS-Nummer:
Molekulargewicht:
272.42
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352211
PubChem Substanz-ID:
NACRES:
NA.25
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (capillary GC)
Lipid-Typ
saturated FAs
Lagertemp.
2-8°C
SMILES String
CCCCCCCCCCCCCCC(O)C(O)=O
InChI
1S/C16H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15(17)16(18)19/h15,17H,2-14H2,1H3,(H,18,19)
InChIKey
JGHSBPIZNUXPLA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
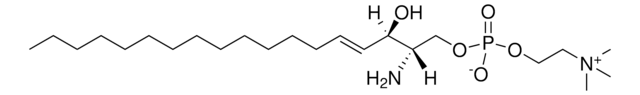
Biochem./physiol. Wirkung
2-Hydroxyhexadecanoic acid (2OH-HDA) is used as a representative of the saturated long-chain hydroxyl fatty acids group, members of which have potential roles in anti-inflammatory action, neuroprotection, and bactericide and anti-cancer defense.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Elena E Pohl et al.
Biochimica et biophysica acta, 1778(5), 1292-1297 (2008-03-04)
Hydroxyl group-containing fatty acids play an important role in anti-inflammatory action, neuroprotection, bactericide and anti-cancer defense. However, the mechanism of long-chain hydroxy fatty acids (HFA) transport across plasma membranes is still disputed. Two main hypotheses have been suggested: firstly, that
Tomotake Morita et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1597-1599 (2011-08-09)
Cryptococcus humicola JCM 1461 efficiently produced cellobiose lipids (CLs), bolaform biosurfactants. The main product was identified as 16-O-(2″,3″,4″,6'-tetra-O-acetyl-β-cellobiosyl)-2-hydroxyhexadecanoic acid. The production yield of CLs reached 13.1 g/L under the intermittent feeding of glucose. The critical micelle concentrations (CMC) of the
J A Hamilton
Prostaglandins, leukotrienes, and essential fatty acids, 60(5-6), 291-297 (1999-09-02)
In early research on fatty acid transport, passive diffusion seemed to provide an adequate explanation for movement of fatty acids through the membrane bilayer. This simple hypothesis was later challenged by the discovery of several proteins that appeared to be
T Kaneshiro et al.
Lipids, 28(5), 397-401 (1993-05-01)
Fumonisin B1 is a sphingolipid-like compound that enhances the accumulation of yeast sphingolipids and 2-hydroxy fatty acids. These lipids occur both as freely extractable and cell bound components in yeast fermentations. Both free and bound 2-hydroxy fatty acids produced by
S Hamanaka et al.
Biochimica et biophysica acta, 961(3), 374-377 (1988-08-12)
Monoglycosylceramides were isolated from pig epidermal cells which had been prepared free from dermal elements. The most polar glycolipid among the five isolated monoglycosylceramides was galactosylceramide. The galactosylceramide was composed of alpha-hydroxypalmitic acid and 16- and 18-carbon chain sphingenine, being
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.