Wichtige Dokumente
G1774
Glucagon
≥95% (HPLC), powder, synthetic
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Sterilität
non-sterile
Assay
≥95% (HPLC)
Form
powder
Methode(n)
cell based assay: suitable
Löslichkeit
1% acetic acid: 1.00-1.04 mg/mL, clear, colorless
water: 1.00-1.04 mg/mL, clear, colorless
Eignung
suitable for molecular biology
UniProt-Hinterlegungsnummer
Versandbedingung
ambient
Lagertemp.
−20°C
SMILES String
Cl[H].[H]N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](C(C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccc(O)cc4)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc5ccccc5)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc6c[nH]c7ccccc67)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)O)C(O)=O
InChI
1S/C153H225N43O49S.ClH/c1-72(2)52-97(133(226)176-96(47-51-246-11)132(225)184-104(60-115(159)209)143(236)196-123(78(10)203)151(244)245)179-137(230)103(58-83-64-167-89-29-19-18-28-87(83)89)183-131(224)95(43-46-114(158)208)177-148(241)120(74(5)6)194-141(234)101(54-79-24-14-12-15-25-79)182-138(231)105(61-117(211)212)185-130(223)94(42-45-113(157)207)171-124(217)75(7)170-127(220)91(31-22-49-165-152(160)161)172-128(221)92(32-23-50-166-153(162)163)174-146(239)110(69-199)191-140(233)107(63-119(215)216)186-134(227)98(53-73(3)4)178-135(228)99(56-81-33-37-85(204)38-34-81)180-129(222)90(30-20-21-48-154)173-145(238)109(68-198)190-136(229)100(57-82-35-39-86(205)40-36-82)181-139(232)106(62-118(213)214)187-147(240)111(70-200)192-150(243)122(77(9)202)195-142(235)102(55-80-26-16-13-17-27-80)188-149(242)121(76(8)201)193-116(210)66-168-126(219)93(41-44-112(156)206)175-144(237)108(67-197)189-125(218)88(155)59-84-65-164-71-169-84;/h12-19,24-29,33-40,64-65,71-78,88,90-111,120-123,167,197-205H,20-23,30-32,41-63,66-70,154-155H2,1-11H3,(H2,156,206)(H2,157,207)(H2,158,208)(H2,159,209)(H,164,169)(H,168,219)(H,170,220)(H,171,217)(H,172,221)(H,173,238)(H,174,239)(H,175,237)(H,176,226)(H,177,241)(H,178,228)(H,179,230)(H,180,222)(H,181,232)(H,182,231)(H,183,224)(H,184,225)(H,185,223)(H,186,227)(H,187,240)(H,188,242)(H,189,218)(H,190,229)(H,191,233)(H,192,243)(H,193,210)(H,194,234)(H,195,235)(H,196,236)(H,211,212)(H,213,214)(H,215,216)(H,244,245)(H4,160,161,165)(H4,162,163,166);1H/t75-,76+,77+,78+,88-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-;/m0./s1
InChIKey
RKGLLHCSSVJTAN-YYICOITRSA-N
Angaben zum Gen
human ... GCG(2641)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
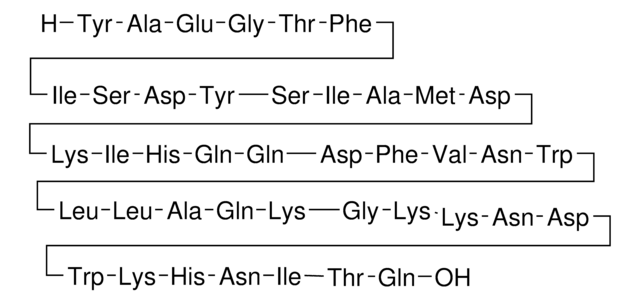
Amino Acid Sequence
Allgemeine Beschreibung
Anwendung
- used as a component of hormone stock solution for preserving full biological activity of heart tissues obtained from male Sprague-Dawley rats
- used as an infusion in phases II and III fasting king penguins to study the various lipolytic, metabolic, and hormonal responses
- used for the stimulation of PGC-1α expression in hepatocytes
- used to induce the expression of methionine adenosyltransferase α1 (MAT1A), which is involved in the regulation of hepatic levels of S-adenosylmethionine and the adaptive response to fasting
- used to study its effect on stimulation of gluconeogenesis through hepatic lipolysis, mediated by inositol trisphosphate receptor 1 (INSP3R1)
- intraperitoneally injected in mice to assess glucagon-induced Sam68 subcellular localization in vivo
Biochem./physiol. Wirkung
Komponenten
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![[Arg8]-Vasopressin solution Grade VI (synthetic), ~100 IU/mL in 0.9% NaCl](/deepweb/assets/sigmaaldrich/product/structures/326/242/dede8c26-cf73-4a28-a5d9-1d57c673cf0e/640/dede8c26-cf73-4a28-a5d9-1d57c673cf0e.png)